Abstract
We demonstrate the first use of a multifibre Raman probe that fits inside the bore of a hypodermic needle. A Raman probe containing multiple collection fibres provides improved signal collection efficiency in biological samples compared with a previous two-fibre design. Furthermore, probe performance (signal-to-noise ratios) compared favourably with the performance achieved in previous Raman microscope experiments able to distinguish between benign lymph nodes, primary malignancies in lymph nodes and secondary malignancies in lymph nodes. The experimental measurements presented here give an indication of the sampling volume of the Raman needle probe in lymphoid tissues. Liquid tissue phantoms were used that contained scattering medium encompassing a range of scattering properties similar to those of a variety of tissue types, including lymph node tissues. To validate the appropriateness of the phantoms, the sampling depth of the probe was also measured in excised lymph node tissue. More than 50 % of Raman photons collected were found to originate from between the tip of the needle and a depth of 500 μm into the tissue. The needle probe presented here achieves spectral quality comparable to that in numerous studies previously demonstrating Raman disease discrimination. It is expected that this approach could achieve targeted subcutaneous tissue measurements and be viable for use for the in vivo Raman diagnostics of solid organs located within a few centimetres below the skin’s surface.

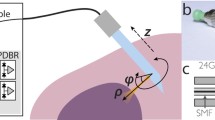
Schematic of multi-fibre Raman needle probe with disposible tips and proximal optical filtration






Similar content being viewed by others
References
Ellis DI, Cowcher DP, Ashton L, O'Hagan S, Goodacre R (2013) Illuminating disease and enlightening biomedicine: Raman spectroscopy as a diagnostic tool. Analyst 138(14):3871–3884
Talari ACS, Movasaghi Z, Rehman S, Rheman IU (2014) Raman spectroscopy of biological tissues. Appl Spectrosc Rev 50:46–111
Carden A, Morris MD (2000) Application of vibrational spectroscopy to the study of mineralized tissues (review). J Biomed Opt 5(3):259–268
Hanlon EB, Manoharan R, Koo T-W, Shafer KE, Motz JT, Fitzmaurice M, Kramer JR, Itzkan I, Dasari RR, Feld MS (2000) Prospects for in vivo Raman spectroscopy. Phys Med Biol 45(2):R1–R59
Stone N, Kendall C, Shepherd N, Crow P, Barr H (2002) Near-infrared Raman spectroscopy for the classification of epithelial pre-cancers and cancers. J Raman Spectrosc 33:564–573
Matousek P, Morris MD, Everall N, Clark IP, Towrie M, Draper E, Goodship A, Parker AW (2005) Numerical simulations of subsurface probing in diffusely scattering media using spatially offset Raman spectroscopy. Appl Spectrosc 59(12):1485–1492
Everall N, Hahn T, Matousek P, Parker AW, Towrie M (2004) Photon migration in Raman spectroscopy. Appl Spectrosc 58(5):591–597
Matousek P, Stone N (2009) Emerging concepts in deep Raman spectroscopy of biological tissue. Analyst 134:1058–1066
Matousek P, Clark IP, Draper ERC, Morris MD, Goodship AE, Everall N, Towrie M, Finney WF, Parker AW (2005) Subsurface probing in diffusely scattering media using spatially offset Raman spectroscopy. Appl Spectrosc 59(4):393–400
Matousek P, Stone N (2008) Advanced transmission Raman spectroscopy: a promising tool for breast disease diagnosis. Cancer Res 68:4424
Matousek P, Everall N, Towrie M, Parker AW (2005) Depth profiling in diffusely scattering media using Raman spectroscopy and picosecond Kerr gating. Appl Spectrosc 59(2):200–205
Iping Petterson IE, Dvořák P, Buijs J, Gooijer C, Ariese F (2010) Time-resolved spatially offset Raman spectroscopy for depth analysis of diffusely scattering layers. Analyst 135(12):3255–3259
Matousek P, Stone N (2013) Recent advances in the development of Raman spectroscopy for deep non-invasive medical diagnosis. J Biophotonics 6(1):7–19
Draga ROP, Grimbergen MCM, Vijverberg PLM, van Swol CFP, Jonges TGN, Kummer JA, Bosch JLHR (2010) In vivo bladder cancer diagnosis by high-volume Raman spectroscopy. Anal Chem 82:5993–5999
Magee ND, Villaumie JS, Marple ET, Ennis M, Elborn JS, McGarvey JJ (2009) Ex vivo diagnosis of lung cancer using a Raman miniprobe. J Phys Chem B 113:8137–8141
Matthäus C, Dochow S, Bergner G, Lattermann A, Romeike BFM, Marple ET, Krafft C, Dietzek B, Brehm BR, Popp J (2012) In vivo characterization of atherosclerotic plaque depositions by Raman-probe spectroscopy and in vitro coherent anti-stokes Raman scattering microscopic imaging on a rabbit model. Anal Chem 84:7845–7851
Agenant M, Grimbergen M, Draga R, Marple E, Bosch R, van Swol C (2014) Clinical superficial Raman probe aimed for epithelial tumor detection: phantom model results. Biomed Opt Express 5(4):1203–1216
Almond LM, Hutchings J, Kendall C, Day JCC, Stevens OAC, Lloyd GR, Shepherd NA, Barr H, Stone N (2012) Assessment of a custom-built Raman spectroscopic probe for diagnosis of early oesophageal neoplasia. J Biomed Opt 17(8):081421
Shim MG, Wilson BC, Marple E, Wach M (1999) Study of fibre-optic probes for in vivo medical Raman spectroscopy. Appl Spectrosc 53(6):619–627
Huang Z, Zeng H, Hamzavi I, McLean DI, Lui H (2001) Rapid near-infrared Raman spectroscopy system for real-time in vivo skin measurements. Opt Lett 26(22):1782–1784
Komachi Y, Katagiri T, Sato H, Tashiro H (2009) Improvement and analysis of a micro Raman probe. Appl Opt 48:1683–1696
Mahadevan-Jansen A, Mitchell MF, Ramanujam N, Utzinger U, Richards-Kortum R (1998) Development of a fiber optic probe to measure NIR Raman spectra of cervical tissue in vivo. Photochem Photobiol 68:427–431
Shim M, Song LMWK, Marcon NE, Wilson BC (2000) In vivo near-infrared Raman spectroscopy: demonstration of feasibility during clinical gastrointestinal endoscopy. Photochem Photobiol 72(1):146–150
Utzinger U, Richards-Kortum RR (2003) Fiber optic probes for biomedical optical spectroscopy. J Biomed Opt 8(1):121–147
Day JCC, Stone N (2013) A subcutaneous Raman needle probe. Appl Spectrosc 67(3):349–354
Cooney TF, Skinner HT, Angel SM (1996) Comparative study of some fiber-optic remote Raman probe designs. Part II: tests of single-fiber, lensed, and flat- and bevel-tip multi-fiber probes. Appl Spectrosc 50(7):849–860
Tai DCS, Hooks DA, Harvey JD, Smaill BH, Soeller C (2007) Illumination and fluorescence collection volumes for fiber optic probes in tissue. J Biomed Opt 12(3):034033
Lloyd GR, Orr LE, Christie-Brown J, McCarthy K, Rose S, Thomas M, Stone N (2013) Discrimination between benign, primary and secondary malignancies in lymph nodes from the head and neck utilising Raman spectroscopy and multivariate analysis. Analyst 138:3900–3908
Horsnell J, Stonelake P, Christie-Brown J, Shetty G, Hutchings J, Kendall C, Stone N (2010) Raman spectroscopy—a new method for the intra-operative assessment of axillary lymph nodes. Analyst 135:3042–3047
Horsnell JD, Smith JA, Sattlecker M, Sammon A, Christie-Brown J, Kendall C, Stone N (2012) Raman spectroscopy—a potential new method for the intra-operative assessment of axillary lymph nodes. Surgeon 10:123–127
The Engineering ToolBox. Smaller circles in larger circles. http://www.engineeringtoolbox.com/smaller-circles-in-larger-circle-d_1849.html. Accessed 9 Dec 2014
Sadhwani A, Schomacker KT, Tearney GJ, Nishioka NS (1996) Determination of Teflon thickness with laser speckle. I. Potential for burn depth diagnosis. Appl Opt 35(28):5727–5735
Iping Petterson IE, Ariese F (2012) Time-resolved Raman spectroscopy for non-invasive detection through non-transparent materials. Spectrosc Eur 24(1):19–21
Aernouts B, Van Beers R, Watté R, Lammertyn J, Saeys W (2014) Dependent scattering in Intralipid® phantoms in the 600-1850 nm range. Opt Express 22(5):6086–6098
Royston DD, Poston RS, Prahl SA (1996) Optical properties of scattering and absorbing materials used in the development of optical phantoms at 1064 nm. J Biomed Opt 1(1):110–116
Di Ninni P, Martelli F, Zaccanti G (2011) Intralipid: towards a diffusive reference standard for optical tissue phantoms. Phys Med Biol 56:N21–N28
Spinelli L, Botwicz M, Zolek N, Kacprzak M, Milej D, Liebert A, Weigel U, Durduran T, Foschum F, Kienle A, Baribeau F, Leclair S, Bouchard J-P, Noiseux I, Gallant P, Mermut O, Pifferi A, Torricelli A, Cubeddu R, Ho H-C, Mazurenka M, Wabnitz H, Klauenberg K, Bodnar O, Elster C, Bénazech-Lavoué M, Bérubé-Lauzière Y, Lesage F, Di Ninni P, Martelli F, Zaccanti G (2012) Inter-laboratory comparison of optical properties performed on intralipid and India ink. In: Biomedical optics and 3-D imaging, OSA technical digest. Optical Society of America, Washington, paper BW1A.6
Pogue BW, Patterson MS (2006) Review of tissue simulating phantoms for optical spectroscopy, imaging and dosimetry. J Biomed Opt 11(4):0411021–04110216
Jacques SL (2013) Optical properties of biological tissues: a review. Phys Med Biol 58:R37–R61
Cubeddu R, Pifferi A, Taroni P, Torricelli A, Valentini G (1997) A solid tissue phantom for photon migration studies. Phys Med Biol 42:1971–1979
Michels R, Foschum F, Kienle A (2008) Optical properties of fat emulsions. Opt Express 16(8):5907
Madsen SJ, Patterson MS, Wilson BC (1992) The use of India ink as an optical absorber in tissue-simulating phantoms. Phys Med Biol 31(4):985–993
Di Ninni P, Martelli F, Zaccanti G (2010) The use of India ink in tissue-simulating phantoms. Opt Express 18(26):26860
Acknowledgments
The authors thank Martha Vardaki for India ink characterisation and Gloucester Hospitals NHS Foundation Trust for providing the excised human lymph node tissue. This work was funded by a UK National Institute for Health Research Invention for Innovation (i4i) grant, number II_LA_1111_20007.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Raman4Clinics with guest editors Jürgen Popp and Christoph Krafft.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 71 kb)
Rights and permissions
About this article
Cite this article
Iping Petterson, I.E., Day, J.C.C., Fullwood, L.M. et al. Characterisation of a fibre optic Raman probe within a hypodermic needle. Anal Bioanal Chem 407, 8311–8320 (2015). https://doi.org/10.1007/s00216-015-9021-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-9021-7