Abstract
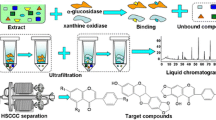
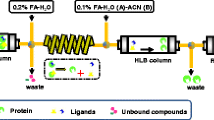
Xanthine oxidase (XOD) catalyzes the metabolism of hypoxanthine and xanthine to uric acid, the overproduction of which could cause hyperuricemia, a risk factor for gout. Inhibition of XOD is a major treatment for gout, and biflavonoids have been found to act as XOD-inhibitory compounds. In this study, ultrafiltration liquid chromatography with photodiode-array detection coupled to electrospray-ionization tandem mass spectrometry (UF-LC-PDA–ESI-MS) was used to screen and identify XOD inhibitors from S. tamariscina. High-performance counter-current chromatography (HPCCC) was used to separate and isolate the active constituents of these XOD inhibitors. Furthermore, ultrahigh-performance liquid chromatography (UPLC) and triple-quadrupole mass spectrometry (TQ-MS) was used to determine the XOD-inhibitory activity of the obtained XOD inhibitors, and enzyme kinetics was performed with Lineweaver–Burk (LB) plots using xanthine as the substrate. As a result, two compounds in S. tamariscina were screened as XOD inhibitors: 65.31 mg amentoflavone and 0.76 mg robustaflavone were isolated from approximately 2.5 g S. tamariscina by use of HPCCC. The purities of the two compounds obtained were over 98 % and 95 %, respectively, as determined by high-performance liquid chromatography (HPLC). Lineweaver–Burk plot analysis indicated that amentoflavone and robustaflavone were non-competitive inhibitors of XOD, and the IC 50 values of amentoflavone and robustaflavone for XOD inhibition were 16.26 μg mL−1 (30.22 μmol L−1) and 11.98 μg mL−1 (22.27 μmol L−1), respectively. The IC 50 value of allopurinol, used as the standard, was 7.49 μg mL−1 (46.23 μmol L−1). The results reveal that the method for systematic screening, identification, and isolation of bioactive components in S. tamariscina and for detecting their inhibitory activity using ultrafiltration LC–ESI-MS, HPCCC, and UPLC–TQ-MS is feasible and efficient, and could be expected to extend to screening and separation of other enzyme inhibitors.

ᅟ




Similar content being viewed by others
References
Dawson J, Quinn T, Walters M (2007) Uric acid reduction: a new paradigm in the management of cardiovascular risk. Curr Med Chem 14:1879–1886
Lin KC, Lin HY, Chou P (2000) The interaction between uric acid level and other risk factors on the development of gout among asymptomatic hyperuricemic men in a prospective study. J Rheumatol 27:1501–1505
Gao X, Qi L, Qiao N, Choi HK, Curhan G, Tucker KL, Ascherio A (2007) Intake of added sugar and sugar-sweetened drink and serum uric acid concentration in US men and women. Hypertension 50:306–312
Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta J, Patel JM, Johnson RJ (2006) A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 290:625–631
Kondo M, Hirano Y, Nishio M, Furuya Y, Nakamura H, Watanabe T (2013) Xanthine oxidase inhibitory activity and hypouricemic effect of aspalathin from unfermented rooibos. J Food Sci 78:1935–1939
Borges F, Fernandes E, Roleira F (2002) Progress towards the discovery of xanthine oxidase inhibitors. Curr Med Chem 9:195–217
Harris MD, Siegel LB, Alloway JA (1999) Gout and hyperuricemia. Am Fam Physician 59:925–934
Liu S, Xing JP, Zheng Z, Song FR, Liu ZQ, Liu SY (2012) Ultrahigh performance liquid chromatography–triple quadrupole mass spectrometry inhibitors fishing assay: a novel method for simultaneously screening of xanthine oxidase inhibitor and superoxide anion scavenger in a single analysis. Anal Chim Acta 715:64–70
Tsai TF, Yeh TY (2010) Allopurinol in dermatology. Am J Clin Dermatol 11:225–232
Arimboor R, Rangan M, Aravind SG, Arumughan C (2011) Tetrahydroamentoflavone (THA) from Semecarpus anacardium as a potent inhibitor of xanthine oxidase. J Ethnopharmacol 133:1117–1120
Kim HP, Park H, Son KH, Chang HW, Kang SS (2008) Biochemical pharmacology of biflavonoids: implications for anti-inflammatory action. Pharm Res 31:265–273
Li DQ, Li SP, Zhao J (2014) Screening of xanthine oxidase inhibitors in complex mixtures using online HPLC coupled with postcolumn fluorescencebased biochemical detection. J Sep Sci 37:338–344
Zhou H, Xing JP, Liu S, Song FR, Cai ZW, Pi ZF, Liu ZQ, Liu SY (2012) Screening and determination for potential xanthine oxidase inhibitors from leaves of Acanthopanax senticosus harms by using UF-LC/MS and ESI-MSn. Phytochem Anal 24:315–323
Yang ZZ, Zhang YF, Sun LJ, Wang Y, Gao XM, Cheng YY (2012) An ultrafiltration high-performance liquid chromatography coupled with diode array detector and mass spectrometry approach for screening and characterizing tyrosinase inhibitors from mulberry leaves. Anal Chim Acta 719:87–95
Lin CM, Chen CS, Chen CT, Liang YC, Lin JK (2002) Molecular modeling of flavonoids that inhibits xanthine oxidase. Biochem Biophys Res Commun 294:167–172
Mahjoub MA, Ammar S, Edziri H, Mighri N, Bouraoui A, Mighri Z (2010)Anti-inflammatory and antioxidant activities of some extracts and pure natural products isolated from Rhus tripartitum (Ucria). Med Chem Res 19:271–282
Adaramoye OA, Medeiros IAJ (2009) Endothelium independent vasodilation induced by kolaviron, a biflavonoid complex from Garcinia kola seeds, in rat superior mesenteric arteries. Smooth Muscle Res 45:39–53
Woo ER, Pokharel YR, Yang JW, Lee SY (2006) Inhibition of nuclear factor-k B activation by 2′,8″-biapigenin. Biol Pharm Bull 29:976–980
Zheng XK, Li YJ, Zhang L, Feng WS, Zhang X (2011) Antihyperglycemic activity of Selaginella tamariscina (Beauv.) Spring. J Ethnopharmacol 133:531–537
Rahman M, Riaz M, Desai UR (2007) Synthesis of biologically relevant biflavanoids–a review. Chem Biodivers 4:2495–2527
Wang J, Bai HL, Liu CM, Li L (2010) Isolation and purification of ginsenosides from plant extract of Panax quinquefolium L. by high performance centrifugal partition chromatography coupled with evaporative light scattering detection. Chromatographia 71:267–271
Yuan ED, Liu BG, Ning ZX, Chen CG (2009) Preparative separation of flavonoids in Adinandra nitida leaves by high-speed counter-current chromatography and their effects on human epidermal carcinoma cancer cells. Food Chem 115:1158–1163
Yuan Y, Wang BQ, Chen LJ, Luo HD, Fisher D, Sutherland IA, Wei YQ (2008) How to realize the linear scale-up process for rapid purification using high-performance counter-current chromatography. J Chromatogr A 1194:192–198
Zhang YX, Li QY, Yan LL, Shi Y (2011) Structural characterization and identification of biflavones in Selaginella tamariscina by liquid chromatography-diode-arraydetection/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 25:2173–2186
Shi SY, Peng MJ, Zhang YP, Peng S (2013) Combination of preparative HPLC and HSCCC methods to separate phosphodiesterase inhibitors from Eucommia ulmoides bark guided by ultrafiltration-based ligand screening. Anal Bioanal Chem 405:4213–4223
Shi SY, Zhou HH, Huang KL (2008) Hyphenated HSCCC-DPPH for rapid preparative isolation and screening of antioxidants from Selaginella moellendorffii. Chromatographia 68:173–178
Romani A, Galardi C, Pinelli P (2002) HPLC quantification of flavonoids and Biflavonoids in Cupressaceae leaves. Chromatographia 56:469–474
Tan WJ, Xu JC, Li L, Chen KL (2009) Bioactive compounds of inhibiting xanthine oxidase from Selaginella labordei. Nat Prod Res 23:393–398
Acknowledgments
This work was supported by the National Natural Science Foundation of China (nos 21175128, 81303280, 31370374, 31373899) and the Natural Science Foundation of Jilin Province (No. 20130413043GH, 20130521013ZH, [2013]253, [2013]254, [2012]224).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 29 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Liu, S., Ma, B. et al. Rapid screening and detection of XOD inhibitors from S. tamariscina by ultrafiltration LC-PDA–ESI-MS combined with HPCCC. Anal Bioanal Chem 406, 7379–7387 (2014). https://doi.org/10.1007/s00216-014-8132-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8132-x