Abstract
Ultraperformance convergence chromatography/tandem triple quadrupole mass spectrometry (UPC2-MS/MS) is a novel tool in separation science that combines the advantages of supercritical fluid chromatography with ultraperformance liquid chromatography/MS/MS technology. The use of nontoxic CO2 fluid and a postcolumn additive to complement MS/MS allows better control of analyte retention for chiral separation and high-sensitivity determination with different chiral stationary phases. This paper reports the stereoselective separation and determination of the chiral neonicotinoid sulfoxaflor in vegetables and soil by UPC2-MS/MS. Baseline resolution (Rs ≥ 1.56) of and high selectivity (LOQ ≤ 1.83 μg/kg) for the four stereoisomers were achieved by postcolumn addition of 1 % formic acid–methanol to a Chiralpak IA-3 using CO2/isopropanol/acetonitrile as the mobile phase at 40 °C, 2,500 psi, and for 6.5 min in electrospray ionization positive mode. Rearranged Van’t Hoff equations afforded the thermodynamic parameters ΔH ο and ΔS ο, which were analyzed to promote understanding of the enthalpy-driven separation of sulfoxaflor stereoisomers. The interday mean recovery, intraday repeatability, and interday reproducibility varied from 72.9 to 103.7 %, from 1.8 to 9.2 %, and from 3.1 to 9.4 %, respectively. The proposed method was used to study the pharmacokinetic dissipation of sulfoxaflor stereoisomers in soil under greenhouse conditions. The estimated half-life ranged from 5.59 to 6.03 d, and statistically nonsignificant enantioselective degradation was observed. This study not only demonstrates that the UPC2-MS/MS system is an efficient and sensitive method for sulfoxaflor stereoseparation, but also provides the first experimental evidence of the pharmacokinetic dissipation of sulfoxaflor stereoisomers in the environment.

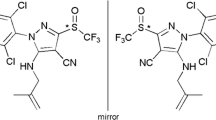
Chemical structure and UPC2-MS/MS separation chromatogram of sulfoxaflor. (* stereogenic center)






Similar content being viewed by others
Abbreviations
- ABPR:
-
Pressure of automated backpressure regulator
- CSP:
-
Chiral stationary phase
- CS-SA:
-
Selector–selectand
- dSPE:
-
Dispersive solid-phase extraction
- EF:
-
Enantiomer fraction
- ESI:
-
Electrospray ionization
- MRL:
-
Maximum residue limit
- MRM:
-
Multiple reaction monitoring
- MWCNTs:
-
Multi-walled carbon nanotubes
- nAChR:
-
Nicotinic acetylcholine receptor
- Rs:
-
Resolution
- RSD:
-
Relative standard deviation
- SSE:
-
Signal suppression and enhancement
References
Zhu Y, Loso MR, Watson GB, Sparks TC, Rogers RB, Huang JX, Gerwick BC, Babcock JM, Kelley D, Hegde VB, Nugent BM, Renga JM, Denholm I, Gorman K, DeBoer GJ, Hasler J, Meade T, Thomas JD (2011) Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap-feeding pests. J Agric Food Chem 59(7):2950–2957
Sparks TC, Watson GB, Loso MR, Geng CX, Babcock JM, Thomas JD (2013) Sulfoxaflor and the sulfoximine insecticides: chemistry, mode of action and basis for efficacy on resistant insects. Pestic Biochem Physiol 107(1):1–7
Longhurst C, Babcock JM, Denholm I, Gorman K, Thomas JD, Sparks TC (2013) Cross-resistance relationships of the sulfoximine insecticide sulfoxaflor with neonicotinoids and other insecticides in the whiteflies Bemisia tabaci and Trialeurodes vaporariorum. Pest Manag Sci 69(7):809–813
Cutler P, Slater R, Edmunds AJ, Maienfisch P, Hall RG, Earley FG, Pitterna T, Pal S, Paul VL, Goodchild J, Blacker M, Hagmann L, Crossthwaite AJ (2013) Investigating the mode of action of sulfoxaflor: a fourth-generation neonicotinoid. Pest Manag Sci 69(5):607–619
Garrison AW (2006) Probing the enantioselectivity of chiral pesticides. Environ Sci Technol 40(1):16–23
Zhang H, Wang X, Zhuang S, Qian M, Jiang K, Wang X, Xu H, Qi P, Wang Q (2012) Enantioselective separation and simultaneous determination of fenarimol and nuarimol in fruits, vegetables, and soil by liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem 404(6–7):1983–1991
Tonon MA, Jabor VA, Bonato PS (2013) Enantioselective analysis of zopiclone in rat brain by liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 405(1):267–273
Babcock JM, Gerwick CB, Huang JX, Loso MR, Nakamura G, Nolting SP, Rogers RB, Sparks TC, Thomas J, Watson GB, Zhu Y (2011) Biological characterization of sulfoxaflor, a novel insecticide. Pest Manag Sci 67(3):328–334
Lysandrou M, Ahmad M, Longhurst C (2010) Comparative efficacy of sulfoxaflor against cotton leafhopper, Amrasca devastans (Distant)(Cicadellidae: Homoptera) under field conditions of punjab and sindh. J Agric Res 48(4):517–524
Siebert M, Thomas J, Nolting S, Leonard B, Gore J, Catchot A, Lorenz G, Stewart S, Cook D, Walton L (2012) Field evaluations of sulfoxaflor, a novel insecticide, against tarnished plant bug (Hemiptera: Miridae) in cotton. J Cotton Sci 16:129–143
Watson GB, Loso MR, Babcock JM, Hasler JM, Letherer TJ, Young CD, Zhu Y, Casida JE, Sparks TC (2011) Novel nicotinic action of the sulfoximine insecticide sulfoxaflor. Insect Biochem Mol Biol 41(7):432–439
Sparks TC, DeBoer GJ, Wang NX, Hasler JM, Loso MR, Watson GB (2012) Differential metabolism of sulfoximine and neonicotinoid insecticides by Drosophila melanogaster monooxygenase CYP6G1. Pestic Biochem Physiol 103(3):159–165
Zhou Q, Gao B, Zhang X, Xu Y, Shi H, Yu LL (2014) Chemical profiling of triacylglycerols and diacylglycerols in cow milk fat by ultra-performance convergence chromatography combined with a quadrupole time-of-flight mass spectrometry. Food Chem 143:199–204
Ministry of Agriculture (2004) NY/T788: Guidelines for pesticide residue field trials. Ministry of Agriculture, People's Republic of China, Beijing
Péter A, Vékes E, Armstrong DW (2002) Effects of temperature on retention of chiral compounds on a ristocetin A chiral stationary phase. J Chromatogr A 958(1):89–107
O’Brien T, Crocker L, Thompson R, Thompson K, Toma P, Conlon D, Feibush B, Moeder C, Bicker G, Grinberg N (1997) Mechanistic aspects of chiral discrimination on modified cellulose. Anal Chem 69(11):1999–2007
Diao J, Xu P, Wang P, Lu Y, Lu D, Zhou Z (2010) Environmental behavior of the chiral aryloxyphenoxypropionate herbicide diclofop-methyl and diclofop: enantiomerization and enantioselective degradation in soil. Environ Sci Technol 44(6):2042–2047
Li Z, Zhang Y, Li Q, Wang W, Li J (2011) Enantioselective degradation, abiotic racemization, and chiral transformation of triadimefon in soils. Environ Sci Technol 45(7):2797–2803
Kažoka H, Koliškina O, Veinberg G, Vorona M (2013) Separation of piracetam derivatives on polysaccharide-based chiral stationary phases. J Chromatogr A 1281:160–165
Khan M, Viswanathan B, Rao DS, Reddy R (2007) Chiral separation of Frovatriptan isomers by HPLC using amylose based chiral stationary phase. J Chromatogr B 846(1):119–123
Ward TJ, Ward KD (2011) Chiral separations: a review of current topics and trends. Anal Chem 84(2):626–635
Zhang T, Nguyen D, Franco P (2008) Enantiomer resolution screening strategy using multiple immobilised polysaccharide-based chiral stationary phases. J Chromatogr A 1191(1):214–222
Ali I, Naim L, Ghanem A, Aboul-Enein HY (2006) Chiral separations of piperidine-2,6-dione analogues on Chiralpak IA and Chiralpak IB columns by using HPLC. Talanta 69(4):1013–1017
Qiu J, Dai S, Zheng C, Yang S, Chai T, Bie M (2011) Enantiomeric separation of triazole fungicides with 3-μm and 5-μml particle chiral columns by reverse-phase high-performance liquid chromatography. Chirality 23(6):479–486
Li J, Dong F, Cheng Y, Liu X, Xu J, Li Y, Chen X, Kong Z, Zheng Y (2012) Simultaneous enantioselective determination of triazole fungicide difenoconazole and its main chiral metabolite in vegetables and soil by normal-phase high-performance liquid chromatography. Anal Bioanal Chem 404(6–7):2017–2031
Sardella R, Ianni F, Lisanti A, Marinozzi M, Scorzoni S, Natalini B (2014) The effect of mobile phase composition in the enantioseparation of pharmaceutically relevant compounds with polysaccharide-based stationary phases. Biomed Chromatogr 28(1):159–167
West C, Bouet A, Routier S, Lesellier E (2012) Effects of mobile phase composition and temperature on the supercritical fluid chromatography enantioseparation of chiral fluoro-oxoindole-type compounds with chlorinated polysaccharide stationary phases. J Chromatogr A 1269:325–335
Berthod A (2006) Chiral recognition mechanisms. Anal Chem 78(7):2093–2099
Lämmerhofer M (2010) Chiral recognition by enantioselective liquid chromatography: mechanisms and modern chiral stationary phases. J Chromatogr A 1217(6):814–856
Wang C, Zhang Y (2013) Effects of column back pressure on supercritical fluid chromatography separations of enantiomers using binary mobile phases on 10 chiral stationary phases. J Chromatogr A 1281:127–134
Abrahamsson V, Rodriguez-Meizoso I, Turner C (2012) Determination of carotenoids in microalgae using supercritical fluid extraction and chromatography. J Chromatogr A 1250:63–68
Wang F, O’Brien T, Dowling T, Bicker G, Wyvratt J (2002) Unusual effect of column temperature on chromatographic enantioseparation of dihydropyrimidinone acid and methyl ester on amylose chiral stationary phase. J Chromatogr A 958(1):69–77
Liu H, Berger SJ, Chakraborty AB, Plumb RS, Cohen SA (2002) Multidimensional chromatography coupled to electrospray ionization time-of-flight mass spectrometry as an alternative to two-dimensional gels for the identification and analysis of complex mixtures of intact proteins. J Chromatogr B 782(1):267–289
Marwah A, Marwah P, Lardy H (2002) Analysis of ergosteroids. VIII: Enhancement of signal response of neutral steroidal compounds in liquid chromatographic–electrospray ionization mass spectrometric analysis by mobile phase additives. J Chromatogr A 964(1–2):137–151
Garcia M (2005) The effect of the mobile phase additives on sensitivity in the analysis of peptides and proteins by high-performance liquid chromatography–electrospray mass spectrometry. J Chromatogr B 825(2):111–123
Corradini D, Huber CG, Timperio AM, Zolla L (2000) Resolution and identification of the protein components of the photosystem II antenna system of higher plants by reversed-phase liquid chromatography with electrospray–mass spectrometric detection. J Chromatogr A 886(1):111–121
Chen Z, Dong F, Xu J, Liu X, Chen Y, Liu N, Tao Y, Zheng Y (2014) Stereoselective determination of a novel chiral insecticide, sulfoxaflor, in brown rice, cucumber and apple by normal-phase high-performance liquid chromatography. Chirality 26(2):114–120
Wang T, Wenslow RM Jr (2003) Effects of alcohol mobile-phase modifiers on the structure and chiral selectivity of amylose tris(3,5-dimethylphenylcarbamate) chiral stationary phase. J Chromatogr A 1015(1):99–110
El-Sheikh AH, Sweileh JA, Al-Degs YS, Insisi AA, Al-Rabady N (2008) Critical evaluation and comparison of enrichment efficiency of multi-walled carbon nanotubes, C18 silica and activated carbon towards some pesticides from environmental waters. Talanta 74(5):1675–1680
Zhao P, Wang L, Zhou L, Zhang F, Kang S, Pan C (2012) Multi-walled carbon nanotubes as alternative reversed-dispersive solid phase extraction materials in pesticide multi-residue analysis with QuEChERS method. J Chromatogr A 1225:17–25
Dong F, Li J, Chankvetadze B, Cheng Y, Xu J, Liu X, Li Y, Chen X, Bertucci C, Tedesco D (2013) The chiral triazole fungicide difenoconazole: absolute stereochemistry, stereoselective bioactivity, aquatic toxicity and environmental behavior in vegetables and soil. Environ Sci Technol 47(7):3386–3394
Zhang H, Wang X, Qian M, Wang X, Xu H, Xu M, Wang Q (2011) Residue analysis and degradation studies of fenbuconazole and myclobutanil in strawberry by chiral high-performance liquid chromatography–tandem mass spectrometry. J Agric Food Chem 59(22):12012–12017
Xu J, Dong F, Liu X, Li J, Li Y, Shan W, Zheng Y (2012) Determination of sulfoxaflor residues in vegetables, fruits and soil using ultra-performance liquid chromatography/tandem mass spectrometry. Anal Methods 4(12):4019
Dong F, Cheng L, Liu X, Xu J, Li J, Li Y, Kong Z, Jian Q, Zheng Y (2012) Enantioselective analysis of triazole fungicide myclobutanil in cucumber and soil under different application modes by chiral liquid chromatography/tandem mass spectrometry. J Agric Food Chem 60(8):1929–1936
Wong CS (2006) Environmental fate processes and biochemical transformations of chiral emerging organic pollutants. Anal Bioanal Chem 386(3):544–558
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (NSFC, 31272071 and 31171879).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, Z., Dong, F., Xu, J. et al. Stereoselective separation and pharmacokinetic dissipation of the chiral neonicotinoid sulfoxaflor in soil by ultraperformance convergence chromatography/tandem mass spectrometry. Anal Bioanal Chem 406, 6677–6690 (2014). https://doi.org/10.1007/s00216-014-8089-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8089-9