Abstract
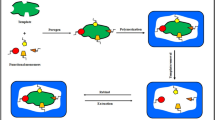
This paper describes the synthesis of novel molecularly imprinted polymers (MIPs), prepared by a noncovalent imprinting approach, for cleanup and preconcentration of curcumin (CUR) and bisdemethoxycurcumin (BDMC) from medicinal herbal extracts and further analysis by high-performance liquid chromatography with fluorescence detection (HPLC-FLD). Two molecular mimics, a mixture of reduced BDMCs and 4-(4-hydroxyphenyl)-2-butanone (HPB), have been synthesized and applied as templates for MIP synthesis. The polymers were prepared using N-(2-aminoethyl) methacrylamide (EAMA) as functional monomer, ethylene glycol dimethacrylate (EDMA) as the cross-linker (in a 1:5 molar ratio), and a mixture of acetonitrile/dimethylsulfoxide (90 %, v/v) as porogen. MIPs prepared using a mixture of reduced BDMCs as template showed higher selectivity for CUR and BDMC than those obtained with HPB, with imprinting factors of 3.5 and 2.7 for CUR and BDMC, respectively, using H2O/acetonitrile (65:35, v/v) as mobile phase. The adsorption isotherms for CUR in the MIP and the nonimprinted polymer (NIP) were fitted to the Freundlich isotherm model, and the calculated average binding affinities for CUR were (17 ± 2) and (8 ± 1) mM−1 for the MIP and the NIP, respectively. The polymers were packed into solid-phase extraction (SPE) cartridges, and the optimized molecularly imprinted solid-phase extraction (MISPE-HPLC) with fluorescence detection (FLD) method allowed the extraction of both curcuminoids from aqueous samples (50 mM NH4Ac, pH 8.8) followed by a selective washing with acetonitrile/NH4Ac, 50 mM at pH 8.8 (30:70 %, v/v), and elution with 3 × 1 mL of MeOH. Good recoveries and precision ranging between 87 and 92 %, with relative standard deviation (RSD) of <5.3 % (n = 3), were obtained after the preconcentration of 10-mL solutions containing both CUR and BDMC at concentrations in the range of 0–500 μg L−1. The optimized method has been applied to the analysis of both curcuminoids in medicinal herbal extracts.

ᅟ




Similar content being viewed by others
References
Jayaprakasha GK, Jaganmohan LR, Sakariah KK (2006) Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem 98:720–724
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2:270–278
Hwan LJ, Myoung-Gun C (2011) Determination of curcuminoid colouring principles in commercial foods by HPLC. Food Chem 124:1217–1222
Priyadarsini KI, Maity DK, Naik GH, Kumar MS, Unnikrishnan MK, Satav JG, Mohan H (2003) Role of phenolic OH and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Radic Biol Med 35:475–484
Pana MH, Ho CT (2008) Chemopreventive effects of natural dietary compounds on cancer development. Chem Soc Rev 37:2558–2574
Baliga MS, Joseph N, Venkataranganna MV, Saxena A, Ponemoned V, Fayad R (2012) Curcumin, an active component of turmeric in the prevention and treatment of ulcerative colitis: preclinical and clinical observations. Food Funct 3:1109–1117
Regulation (EC) 1333/2008 of the European Parliament and of the Council (2008) On food additives. Off J Eur Union L 354:16–33
http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-additives/en/ (Accessed 26 Dec 2013)
Mahattanadul S, Nakamura T, Panichayupakaranant P, Phdoongsombut N, Tungsinmunkong K, Booking P (2009) Comparative antiulcer effect of bisdemethoxycurcumin and curcumin in a gastric ulcer model system. Phytomed 16:342–351
Thongcai W, Liawruangrath B, Liawruangrath S (2009) Flow injection analysis of total curcuminoids in turmeric and total antioxidant capacity using 2,2′-diphenyl-1-picrylhydrazyl assay. Food Chem 112:494–499
Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB (2008) Biological activities of curcumin and its analogues (Congeners) made by man and mother nature. Biochem Pharmacol 76:1590–1611
Gupta AP, Gupta MM, Sushil K (1999) Simultaneous determination of curcuminoids in Curcuma samples using high performance thin layer chromatography. J Liq Chromatogr Relat Technol 22:1561–1569
Jadhav BK, Mahadik KR, Paradkar AR (2007) Development and validation of improved reversed phase-HPLC method for simultaneous determination of curcumin, demethoxycurcumin and bis-demethoxycurcumin. Chromatogr 65:483–488
Heath DD, Pruitt MA, Brenner DE, Rock CL (2003) Curcumin in plasma and urine: quantitation by high-performance liquid chromatography. J Chromatogr B 783:287–295
Lin XL, Xue L, Zhang HY, Zhu CF (2005) Determination of curcumins in turmeric by micellar electrokinetic capillary chromatography. Can J Anal Sci Spectrosc 51:35–42
Rohman A (2012) Analysis of curcuminoids in food and pharmaceutical products. Int Food Res J 19:19–27
Tóth B, Horvai G (2012) Chromatography, solid-phase extraction, and capillary electrochromatography with MIPs. In: Haupt K (ed) Molecular imprinting, 325th edn. Springer, Berlin, pp 267–306
Wang P, Hu W, Su W (2008) Molecularly imprinted poly(methacrylamide-co-methacrylic acid) composite membranes for recognition of curcumin. Anal Chim Acta 615:54–62
Azizi ES, Ahmad MN, Islam AKMS, Arbain D, Tahir I (2011) Porogen effect towards the quality of curcumin imprinted polymer. J Chem 11:207–211
Kitabatake T, Tabo H, Matsunaga H, Haginaka J (2013) Preparation of monodisperse curcumin-imprinted polymer by precipitation polymerization and its application for the extraction of curcuminoids from Curcuma longa L. Anal Bioanal Chem 405:6555–6561
Piletska EV, Burns R, Terry LA, Piletsky SA (2012) Application of a molecularly imprinted polymer for extraction of kukoamine A from potato peels. J Agr Food Chem 60:95–99
Vasapollo G, Del Sole R, Mergola L, Lazzoi MR, Scardino A, Scorrano S, Mele G (2011) Molecularly imprinted polymers: present and future prospective. Int J Mol Sci 12:5908–5945
Chen L, Xu S, Li J (2011) Recent advances in molecular imprinting technology: current status, challenges and highlighted applications. Chem Soc Rev 40:2922–2942
Urraca JL, Marazuela MD, Merino ER, Orellana G, Moreno-Bondi MC (2006) Molecularly imprinted polymers with streamlined mimic for zearalenone analysis. J Chromatogr A 1116:127–134
Chen Z, Alvarez-Perez M, Navarro-Villoslada F, Moreno-Bondi MC, Orellana G (2014) Fluorescent sensing of “quat” herbicides with a multifunctional pyrene-labeled monomer and molecular imprinting. Sens Actuat B 191:137–142
Connors KA (1987) Binding constants: the measurement of molecular complex stability. Wiley, New York
Rampey AM, Umpleby RJ, Rushton GT, Iseman JC, Shah RN, Shimizu KD (2004) Characterization of the imprint effect and the influence of imprinting conditions on affinity, capacity and heterogeneity in molecularly imprinted polymers using the Freundlich isotherm-affinity distribution analysis. Anal Chem 76:1123–1133
Ravindran PN, Babu KN, Sivaraman K (eds) (2007) Turmeric: the genus Curcuma. CRC, Boca Raton, p 302
Poole CF, Poole SK (2002) In: Simpson NJK (ed) In: Theory meets practice. Solid phase extraction: principles, techniques and applications. Marcel Dekker Inc, New York, pp 183–226
Acknowledgments
This work has been funded by I-MHERE B.2c grant for Sandwich Doctoral Program of Institut Teknologi Bandung, Indonesia, and by MINECO (ref. CTQ2012-37573-C02-02). JUR thanks the International Excellence Campus CEI-Moncloa for a postdoctoral contract, and ABD thanks the Spanish Ministry of Economy and Competitiveness for a Ramón y Cajal contract.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection celebrating ABCs 13th Anniversary.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 123 KB)
Rights and permissions
About this article
Cite this article
Wulandari, M., Urraca, J.L., Descalzo, A.B. et al. Molecularly imprinted polymers for cleanup and selective extraction of curcuminoids in medicinal herbal extracts. Anal Bioanal Chem 407, 803–812 (2015). https://doi.org/10.1007/s00216-014-8011-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8011-5