Abstract
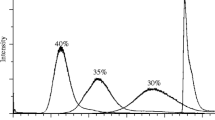
We show here that baseline separation of dansylated estrone, 17β-estradiol, and 17α-estradiol can be done, contrary to previous reports, within a short run time on a single RP-LC analytical column packed with particles bonded with phenyl-hexyl stationary phase. The chromatographic method coupled with isotope dilution tandem MS offers a simple assay enabling the simultaneous analysis of these analytes. The method employs 13C-labeled estrogens as internal standards to eliminate potential matrix effects arising from the use of deuterated estrogens. The assay also offers adequate accuracy and sensitivity to be useful for biological samples. The practical applicability of the validated method is demonstrated by the quantitative analyses of in vivo samples obtained from rats treated with Premarin®.

Quantification of estrogens from rat samples by LC–MS/MS




Similar content being viewed by others
References
Hobe G, Schön R, Goncharov N, Katsiya G, Koryakin M, Gesson-Cholat I, Oettel M, Zimmermann H (2002) Steroids 67:883–893. doi:10.1016/S0039-128X(02)00058-2
Hersh AL, Stefanick ML, Stafford RS (2004) J Am Med Assoc 291:47–53. doi:10.1001/jama.291.1.47
Toran-Allerand CD, Tinnikov AA, Singh RJ, Nethrapalli IS (2005) Endocrinology 146:3843–3850. doi:10.1210/en.2004-1616
Labrie F, Luu-The V, Lin SX, Labrie C, Simard J, Breton R, Bélanger A (1997) Steroids 62:148–158. doi:10.1016/S0039-128X(96)00174-2
Edwards DP, McGuire WL (1980) Endocrinology 107:884–891. doi:10.1210/endo-107-4-884
Clark JH, Williams M, Upchurch S, Eriksson H, Helton E, Markaverich BM (1982) J Steroid Biochem 16:323–328. doi:10.1016/0022-4731(82)90184-4
Ayan D, Roy J, Maltais R, Poirier D (2011) J Steroid Biochem Mol Biol 127:324–330. doi:10.1016/j.jsbmb.2011.07.009
Owens JW, Ashby J (2002) Crit Rev Toxicol 32:445–520. doi:10.1080/20024091064291
Blair IA (2010) Steroids 75:297–306. doi:10.1016/j.steroids.2010.01.012
Tomšíková H, Aufartová J, Solich P, Sosa-Ferrera Z, Santana-Rodríguez JJ, Nováková L (2012) Trends Analyt Chem 34:35–58. doi:10.1016/j.trac.2011.11.008
Giese RW (2003) J Chromatogr A 1000:401–412. doi:10.1016/S0021-9673(03)00306-6
Hu R, Zhang L, Yang Z (2008) Anal Bioan Chem 390:349–359. doi:10.1007/s00216-007-1683-3
Tai SS, Welch MJ (2005) Anal Chem 77:6359–6363. doi:10.1021/ac050837i
Fiers T, Casetta B, Bernaert B, Vandersypt E, Debock M, Kaufman JM (2012) J Chromatogr B 893–894:57–62. doi:10.1016/j.jchromb.2012.02.034
Grover DP, Zhang ZL, Readman JW, Zhou JL (2009) Talanta 78:1204–1210. doi:10.1016/j.talanta.2008.12.049
Prokai-Tatrai K, Bonds D, Prokai L (2010) Chromatographia 71:311–315. doi:10.1365/s10337-009-1441-00009-5893/10/02
Kushnir MM, Rockwood AL, Bergquist J, Varshavsky M, Roberts WL, Yue B, Bunker AM, Meikle AW (2008) Am J Clin Pathol 129:530–539. doi:10.1309/LC03BHQ5XJPJYEKG
Nelson RE, Grebe SK, O'Kane DJ, Singh RJ (2004) Clin Chem 50:373–384. doi:10.1373/clinchem.2003.025478
Couchman L, Vincent RP, Ghataore L, Moniz CF, Taylor NF (2011) Bioanalysis 3:2549–2572. doi:10.4155/bio.11.254
Stanczyk FZ, Clarke NJ (2010) J Steroid Biochem Mol Biol 121:491–495. doi:10.1016/j.jsbmb.2010.05.001
Nguyen HP, Li L, Gatson JW, Maass D, Wigginton JG, Simpkins JW, Schug KA (2011) J Pharm Biomed Anal 54:830–837. doi:10.1016/j.jpba.2010.11.014
Tong X, Ita IE, Wang J, Pivnichny JV (1999) J Pharm Biomed Anal 20:773–784. doi:10.1016/S0731-7085(99)00079-5
Constanzer ML, Chavez-Eng CM, Fu I, Woolf EJ, Matuszewski BK (2005) J Chromatogr B 816:297–308. doi:10.1016/j.jchromb.2004.11.049
Matějíček D (2011) J Chromatogr A 1218:2292–2300. doi:10.1016/j.chroma.2011.02.041
Prokai L, Szarka S, Wang X, Prokai-Tatrai K (2012) J Chromatogr A 1232:281–287. doi:10.1016/j.chroma.2012.01.067
Wang S, Cyronak M, Yang EJ (2007) Pharm Biomed Anal 43:701–707. doi:10.1016/j.jpba.2006.08.010
Hewavitharana AK (2011) J Chromatogr A 1218:359–361. doi:10.1016/j.chroma.2010.11.047
US Department of Health and Human Services, FDA, CDER, CVM (2001) Guidance for industry: bioanalytical method validation. US Food and Drug Administration, Rockville
Snyder LR, Kirkland JJ, Dolan JW (2010) Introduction to modern liquid chromatography, 3rd edn. Wiley, Hoboken
Turowski M, Yamakawa N, Meller J, Kimata K, Ikegami T, Hosoya K, Tanaka N, Thornton ER (2003) J Am Chem Soc 125:13836–13849. doi:10.1021/ja036006g
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (2005) ICH harmonised tripartite guideline. Validation of analytical procedures: text and methodology Q2[R1]. ICH, Geneva
Beránek J, Muggli DA, Kubátová A (2010) J Am Soc Mass Spectrom 21:592–602. doi:10.1016/J.JASMS.2009.12.009
MacCoss MJ, Matthews DE (2005) Anal Chem 77:294A–302A. doi:10.1021/ac053431e
Prokai L, Fryčák P, Stevens SM Jr, Nguyen V (2008) Chromatographia 68:S101–S105. doi:10.1365/s10337-008-0697-0
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Anal Chem 75:3019–3030. doi:10.1021/ac020361s
Prokai-Tatrai K, Prokai L (2011) Methods Mol Biol 789:313–336. doi:10.1007/978-1-61779-310-3_21
Fekete S, Oláh E, Fekete J (2012) J Chromatogr A 1228:57–71. doi:10.1016/j.chroma.2011.09.050
Acknowledgments
We thank Ms. Shastazia White for her excellent technical assistance. This work was supported in part by the National Institutes of Health (grant number AG031535 to LP and AG031421 to KPT) and the Robert A. Welch Foundation (endowment BK-0031 to LP).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Szarka, S., Nguyen, V., Prokai, L. et al. Separation of dansylated 17β-estradiol, 17α-estradiol, and estrone on a single HPLC column for simultaneous quantitation by LC–MS/MS. Anal Bioanal Chem 405, 3399–3406 (2013). https://doi.org/10.1007/s00216-013-6710-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-6710-y