Abstract
Dabigatran etexilate (DABE) is an oral prodrug that is rapidly converted by esterases to dabigatran (DAB), a direct inhibitor of thrombin. To elucidate the esterase-mediated metabolic pathway of DABE, a high-performance liquid chromatography/mass spectrometry based metabolite identification and semi-quantitative estimation approach was developed. To overcome the poor full-scan sensitivity of conventional triple quadrupole mass spectrometry, precursor–product ion pairs were predicted to search for the potential in vitro metabolites. The detected metabolites were confirmed by the product ion scan. A dilution method was introduced to evaluate the matrix effects on tentatively identified metabolites without chemical standards. Quantitative information on detected metabolites was obtained using “metabolite standards” generated from incubation samples that contain a high concentration of metabolite in combination with a correction factor for mass spectrometry response. Two in vitro metabolites of DABE (M1 and M2) were identified, and quantified by the semi-quantitative estimation approach. It is noteworthy that CES1 converts DABE to M1 while CES2 mediates the conversion of DABE to M2. M1 and M2 were further metabolized to DAB by CES2 and CES1, respectively. The approach presented here provides a solution to a bioanalytical need for fast identification and semi-quantitative estimation of CES metabolites in preclinical samples.

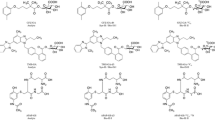
The scheme of the semi-quantitative estimation approach




Similar content being viewed by others
References
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361:1139–1151
Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W (2008) The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 36(2):386–399
Imai T, Taketani M, Shii M, Hosokawa M, Chiba K (2006) Substrate specificity of carboxylesterase isozymes and their contribution to hydrolase activity in human liver and small intestine. Drug Metab Dispos 34(10):1734–1741
Sato Y, Miyashita A, Iwatsubo T, Usui T (2012) Simultaneous absolute protein quantification of carboxylesterases 1 and 2 in human liver tissue fractions using liquid chromatography-tandem mass spectrometry. Drug Metab Dispos 40(7):1389–1396
Delavenne X, Moracchini J, Laporte S, Mismetti P, Basset T (2012) UPLC MS/MS assay for routine quantification of dabigatran—a direct thrombin inhibitor—in human plasma. J Pharm Biomed Anal 58:152–156
Hu Z, Sun Y, Du F, Niu W, Xu F, Huang Y, Li C (2011) Accurate determination of the anticancer prodrug simmitecan and its active metabolite chimmitecan in various plasma samples based on immediate deactivation of blood carboxylesterases. J Chromatogr A 1218:6646–6653
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem 75:3019–3030
Wang J, Williams ET, Bourgea J, Wong YN, Patten CJ (2011) Characterization of recombinant human carboxylesterases: fluorescein diacetate as a probe substrate for human carboxylesterase 2. Drug Metab Dispos 39(8):1329–1333
Hernando MD, Suárez-Barcena JM, Bueno MJ, Garcia-Reyes JF, Fernández-Alba AR (2007) Fast separation liquid chromatography-tandem mass spectrometry for the confirmation and quantitative analysis of avermectin residues in food. J Chromatogr A 1155(1):62–73
Lee HB, Peart TE, Svoboda ML (2007) Determination of ofloxacin, norfloxacin, and ciprofloxacin in sewage by selective solid-phase extraction, liquid chromatography with fluorescence detection, and liquid chromatography–tandem mass spectrometry. J Chromatogr A 1139(1):45–52
Ibáñez M, Sancho JV, Hernández F (2009) Determination of melamine in milk-based products and other food and beverage products by ion-pair liquid chromatography-tandem mass spectrometry. Anal Chim Acta 649(1):91–97
García-Valcárcel AI, Tadeo JL (2009) A combination of ultrasonic assisted extraction with LC-MS/MS for the determination of organophosphorus pesticides in sludge. Anal Chim Acta 641(1–2):117–123
Kruve A, Leito I, Herodes K (2009) Combating matrix effects in LC/ESI/MS: the extrapolative dilution approach. Anal Chim Acta 651(1):75–80
Ferrer C, Lozano A, Agüera A, Girón AJ, Fernández-Alba AR (2011) Overcoming matrix effects using the dilution approach in multiresidue methods for fruits and vegetables. J Chromatogr A 1218(42):7634–7639
Stahnke H, Kittlaus S, Kempe G, Alder L (2012) Reduction of matrix effects in liquid chromatography–electrospray ionization-mass spectrometry by dilution of the sample extracts: how much dilution is needed? Anal Chem 84(3):1474–1482
Vogeser M, Seger C (2010) Pitfalls associated with the use of liquid chromatography–tandem mass spectrometry in the clinical laboratory. Clin Chem 56(8):1234–1244
Yi P, Luffer-Atlas D (2010) A radiocalibration method with pseudo internal standard to estimate circulating metabolite concentrations. Bioanalysis 2:1195–1210
Yu C, Chen CL, Gorycki FL, Neiss TG (2007) A rapid method for quantitatively estimating metabolites in human plasma in the absence of synthetic standards using a combination of liquid chromatography/mass spectrometry and radiometric detection. Rapid Commun Mass Spectrom 21:497–502
Yang Y, Grubb MF, Luk CE, Humphreys WG, Josephs JL (2011) Quantitative estimation of circulating metabolites without synthetic standards by ultra-high-performance liquid chromatography/high resolution accurate mass spectrometry in combination with UV correction. Rapid Commun Mass Spectrom 25:3245–3251
Vishwanathan K, Babalola K, Wang J, Espina R, Yu L, Adedoyin A, Talaat R, Mutlib A, Scatina J (2009) Obtaining exposures of metabolites in preclinical species through plasma pooling and quantitative NMR: addressing metabolites in safety testing (MIST) guidance without using radiolabeled compounds and chemically synthesized metabolite standards. Chem Res Toxicol 22:311–322
Espina R, Yu L, Wang J, Tong Z, Vashishtha S, Talaat R, Scatina J, Mutlib A (2009) Nuclear magnetic resonance spectroscopy as a quantitative tool to determine the concentrations of biologically produced metabolites: implications in metabolites in safety testing. Chem Res Toxicol 22:299–310
Srivastava A, Lian LY, Maggs JL, Chaponda M, Pirmohamed M, Williams DP, Park BK (2010) Quantifying the metabolic activation of nevirapine in patients by integrated applications of NMR and mass spectrometries. Drug Metab Dispos 38:122–132
Valaskovic GA, Utley L, Lee MS, Wu JT (2006) Ultra-low flow nanospray for the normalization of conventional liquid chromatography/mass spectrometry through equimolar response: standard-free quantitative estimation of metabolite levels in drug discovery. Rapid Commun Mass Spectrom 20:1087–1096
Chalcraft KR, Lee R, Mills C, Britz-McKibbin P (2009) Virtual quantification of metabolites by capillary electrophoresis–electrospray ionization–mass spectrometry: predicting ionization efficiency without chemical standards. Anal Chem 81:2506–2515
Acknowledgements
This study was financially supported by grant R15GM096074 from the National Institute of General Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 196 kb)
Rights and permissions
About this article
Cite this article
Hu, ZY., Parker, R.B., Herring, V.L. et al. Conventional liquid chromatography/triple quadrupole mass spectrometry based metabolite identification and semi-quantitative estimation approach in the investigation of in vitro dabigatran etexilate metabolism. Anal Bioanal Chem 405, 1695–1704 (2013). https://doi.org/10.1007/s00216-012-6576-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6576-4