Abstract
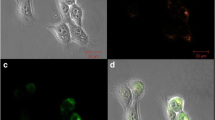
This article presents a dose–response study of the effects of two types of third-generation (G3) and fourth-generation poly(amidoamine) (PAMAM) dendrimers on two cell lines (RTG-2 and H4IIE) by in vitro cytotoxicity assays with 3-(4,5-dimethylthizol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), neutral red uptake (NRU), and lactate dehydrogenase (LDH) assays. We particularly investigated the potential cytotoxic effect of positive surface charge, which a cationic amino-terminated PAMAM dendrimer can display, on the marked ability of PAMAM dendrimers to cross the cell membrane compared with PAMAM dendrimers functionalized with chains of N-(2-hydroxydodecyl). Quantification of dose–response effects was performed by use of mass spectrometry analysis. The analytical method using liquid chromatography–hybrid quadrupole/time-of-flight mass spectrometry that we developed allowed characterization of defective dendrimers instead of “ideal structures.” Identification was based on accurate mass measurement, assignment of elemental composition, and the fully resolved 13 C/12 C isotopic clusters of the multiply charged ions of PAMAM dendrimers. Validation of the liquid chromatography–mass spectrometry method made possible reliable and reproducible quantification of the extracellular and intracellular concentration of dendrimers at a micromolar level (limits of detection from 0.14 to 1.34 μM and from 0.43 to 1.82 μM in standard and culture medium, respectively). A higher cytotoxicity was found with the H4IIE cell line for surface-modified PAMAM dendrimers. The LDH assay was significantly more sensitive than the MTT and NRU assays, with half-maximal inhibitory concentrations (IC50) of 12.96 and 38.31 μg mL-1 for surface-modified G3 and G4 dendrimers, respectively. No cytotoxic effects, in terms of IC50, of amino-terminated PAMAM dendrimers were observed on both H4IIE and RTG-2 cells when the concentration was below 500 μg mL-1 for G3 and G4 dendrimers.




Similar content being viewed by others
References
Project on Emerging Nanotechnologies (2006) Nanotechnology consumer products inventory. http://www.nanotechproject.org/inventories/consumer/. Accessed Apr 2012
Menjoge AR, Kannan RM, Tomalia DA (2010) Drug Discov Today 15:171–185
Klajnert B, Bryszewska M (2007) Dendrimers in medicine. Nova, New York
Ulaszewska MM, Hernando MD, Uclés A, Rosal R, Rodríguez A, García E, Fernández-Alba AR (2012) In: Barceló D, Farré M (eds) Analysis and risk of nanomaterials in environmental and food samples, 1st edn. Elsevier, Amsterdam
Yellepeddi VK, Kumar A, Palakurthi S (2009) Expert Opin Drug Deliv 6:835–850
Naha PC, Davoren M, Casey A, Byrne HJ (2009) Environ Sci Technol 43:6864–6869
Mukherjee SP, Davoren M, Byrne HJ (2010) Toxicol In Vitro 24:169–177
Giri J, Diallo MS, Goddard WA, Dalleska NF, Fang X, Tang Y (2009) Environ Sci Technol 43:5123–5129
Mullen DG, Borgmeier EL, Desai AM (2010) Chemistry 16:10675–10678
Cason CA, Fabré TA, Buhrlage A, Haik KL, Bullen HA (2012) Int J Anal Chem. doi:10.1155/2012/341260
Caminade AM, Laurent R, Majoral JP (2005) Adv Drug Deliv Rev 57:2130–2146
Giordanengo R, Mazarin M, Wu J, Peng L, Charles L (2007) Int J Mass Spectrom 266:62–75
Schwartz BL, Rockwood AL, Smith RD, Tomalia DA, Spindler R (1995) Rapid Commun Mass Spectrom 9:1552–1555
Blasco C, Picó Y (2011) Trends Anal Chem 30:84–99
Jain K, Kesharwani P, Gupta U, Jain NK (2010) Int J Pharm 394:122–142
Mosmann T (1983) J Immunol Methods 65:55–63
Borenfreund E, Puerner JA (1985) Toxicol Lett 24:119–124
Brown DM, Wilson MR, Macnee W, Stone V, Donaldson K (2001) Toxicol Appl Pharmacol 175:191–199
Segner H (2004) Altern Lab Anim 32:375–382
Hong S, Bielinska AU, Mecke A, Keszler B, Beals JL, Shi X, Balogh L, Orr BG, Baker JR, Banaszak Holl MM (2004) Bioconjug Chem 15:774–782
Metullio L, Ferrone M, Coslanich A, Fuchs S, Fermeglia M, Paneni MS, Pricl S (2004) Biomacromolecules 5:1371–1378
Zhou L, Russell DH, Zhao M, Crooks RM (2001) Macromolecules 34:3567–3573
Kallos GJ, Tomalia DA, Hedstrand DM, Lewis S, Zhou J (1991) Rapid Commun Mass Spectrom 5:383–386
Hernando MD, Agüera A, Fernández-Alba AR (2007) Anal Bioanal Chem 387:1269–1285
Janaszewska A, Mączyńska K, Matuszko G, Appelhans D, Voit B, Klajnert B, Bryszewska M (2012) New J Chem 36:428–437
Parimi S, Barnes TJ, Callen DF, Prestidge CA (2010) Biomacromolecules 11:382–389
Saovapakhiran A, D’Emanuele A, Attwood D, Penny J (2009) Bioconjug Chem 20:693–701
Acknowledgments
We thank the Spanish Ministry of Education and Science for financial support through the project “NANOQUAL, Nanoparticles and Water Quality” (National Plan for Scientific Research, Development and Technological Innovation, 2008–2011). M.M.U. acknowledges a research fellowship from the Marie Curie Actions (FP7).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Emerging Contaminants in Biota with guest editors Yolanda Picó and Damià Barceló.
Rights and permissions
About this article
Cite this article
Hernando, M.D., Rosenkranz, P., Ulaszewska, M.M. et al. In vitro dose–response effects of poly(amidoamine) dendrimers [amino-terminated and surface-modified with N-(2-hydroxydodecyl) groups] and quantitative determination by a liquid chromatography–hybrid quadrupole/time-of-flight mass spectrometry based method. Anal Bioanal Chem 404, 2749–2763 (2012). https://doi.org/10.1007/s00216-012-6256-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6256-4