Abstract
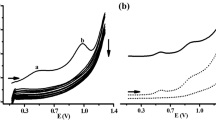
In this work, we have characterized a diamine oxidase (DAO) from Lathyrus sativus and evaluated its use, for the first time, as biocatalytic component of an electrochemical biosensor for the determination of biogenic amines index in wine and beer samples. Firstly, DAO was electrokinetically characterized free in solution by means of a platinum electrode and then immobilized by using polyazetidine prepolimer on the surface of screen-printed electrodes constituted of two gold working electrodes. The amperometric measurements were carried out by using a flow system at a fixed potential of +600 mV vs the internal silver pseudo reference in phosphate buffer solution (0.1 mol l-1, pH = 7.4). The analysis of wine and beer samples were performed in flow injection system using the dual channel transducer providing simultaneous detection of sample and blank signal, and the resulting signal (after subtraction of the blank signal) was referred to that of putrescine. The results were compared with those obtained using a modified reference method based on gas chromatography-mass spectrometry analysis on the same samples. The results obtained in the analysis of Italian wines shows the better suitability of DAO-based biosensor in the determination of the biogenic amines (BAs) index expressed as putrescine equivalent in both red and white wines, being less efficient in beer samples where it underestimates by about 50% the BAs content.





Similar content being viewed by others
References
Silla Santos MH (1996) Biogenic amines: their importance in foods. Int J Food Microbiol 29:213–231
Halásza A, Barátha Á, Simon-Sarkadib L, Holzapfelc W (1994) Biogenic amines and their production by microorganism in food. Trends Food Sci Technol 5:42–49
Shalaby AR (1996) Significance of biogenic amines to food safety and human health. Food Res Int 29:675–690
Biegański T, Kusche J, Lorenz W, Hesterberg R, Stahlknecht C-D, Feussner K-D (1983) Distribution and properties of human intestinal diamine oxidase and its relevance for the histamine catabolism. Biochim et Biophys Acta (BBAs)-Gen Subj 756:196–203
Hasan F, McCrodden JM, Kennedy NP, Tipton KF (1988) The involvement of intestinal monoamine oxidase in the transport and metabolism of tyramine. J Neural Transm Suppl 26:1–9
Horwitz D, Lovenberg W, Engelman K, Sjoerdsma A (1964) Monoamine oxidase inhibitors, tyramine, and cheese. J Am Med Assoc 188:1108–1110
Hui JY, Taylor SL (1985) Inhibition of in vivo histamine metabolism in rat by foodborne and pharmacologic inhibitors of diamine oxidase, histamine N-methyltransferase, and monoamine oxidase. Toxicol Appl Pharmacol 81:241–249
Johnston JP (1968) Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol 17:1285–1297
Hernández-Orte P, Peña-Gallego A, Ibarz MJ, Cacho J, Ferreira V (2006) Determination of the biogenic amines in musts and wines before and after malolactic fermentation using 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate as the derivatizing agent. J Chromatogr A 1129:160–164
Pramateftaki PV, Metafa M, Kallithraka S, Lanaridis P (2006) Evolution of malolactic bacteria and biogenic amines during spontaneous malolactic fermentations in a Greek winery. Lett Appl Microbiol 43:155–160
Manfroi L, Silva PHA, Rizzon LA, Sabaini PS, Glória MBA (2009) Influence of alcoholic and malolactic starter cultures on bioactive amines in Merlot wines. Food Chem 116:208–213
Izquierdo-Pulido M, Font-Fábregas J, Vidal-Carou C (1995) Influence of Saccharomyces cerevisiae var. uvarum on histamine and tyramine formation during beer fermentation. Food Chem 54:51–54
Canás PMI, Romero EG, Alonso SG, González MF, Herreros MLLP (2008) Amino acids and biogenic amines during spontaneous malolactic fermentation in Tempranillo red wines. J Food Compos Anal 21:731–735
Kalač P, Šavel J, Křížek M, Pelikánová T, Prokopová M (2002) Biogenic amine formation in bottled beer. Food Chem 79:431–434
González-Marco A, Ancín-Azpilicueta C (2006) Influence of lees contact on evolution of amines in Chardonnay wine. J Food Sci 71:C544–C548
Marco AG, Azpilicueta CA (2006) Amine concentrations in wine stored in bottles at different temperatures. Food Chem 99:680–685
Lonvaud-Funel A, Joyeux A (1994) Histamine production by wine lactic acid bacteria: isolation of a histamine-producing strain of Leuconostoc oenos. J Appl Microbiol 77:401–407
Önal A (2007) A review: current analytical methods for the determination of biogenic amines in foods. Food Chem 103:1475–1486
Ertan Anli R, Bayram M (2009) Biogenic amines in wines. Food Rev Int 25:86–102
Karoum F, Cattabeni F, Costa E, Ruthven CRJ (1972) Gas chromatographic assay of picomole concentrations of biogenic amines. Anal Biochem 47:550–561
Davis BA, Durden DA, Boulton AA (1986) Simultaneous analysis of twelve biogenic amine metabolites in plasma, cerebrospinal fluid and urine by capillary column gas chromatography-high resolution mass spectrometry with selected-ion monitoring. J Chromatogr B Biomed Sci Appl 374:227–238
Landete GM, Ferrer S, Polo L, Pardo I (2005) Biogenic amines in wines from three Spanish regions. J Agric Food Chem 53:1119–1124
Bover-Cid S, Iquierdo-Pulido M, Mariné-Font A, Vidal-Carou MC (2006) Biogenic mono-, di- and polyamine contents in Spanish wines and influence of a limited irrigation. Food Chem 96:43–47
Romero R, Sánchez-Viñas M, Gázquez D, Bagur MG (2002) Characterization of selected Spanish table wine samples according to their biogenic amine content from liquid chromatographic determination. J Agric Food Chem 50:4713–4717
Kalač P, Hlavatá V, Křížek M (1997) Concentrations of five biogenic amines in Czech beers and factors affecting their formation. Food Chem 58:209–214
Torrea D, Ancían C (2002) Content of biogenic amines in a chardonnay wine obtained through spontaneous and inoculated fermentations. J Agric Food Chem 50:4895–4899
Loukou Z, Zotou A (2003) Determination of biogenic amines as dansyl derivatives in alcoholic beverages by high-performance liquid chromatography with fluorimetric detection and characterization of the dansylated amines by liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. J Chromatogr A 996:103–113
Bauza T, Blak A, Daumas F, Cabani JC (1995) Determination of biogenic amines and their precursor amino acids in wines of the Vallée du Rhône by high-performance liquid chromatography with precolumn derivatization and fluorimetric detection. J Chromatogr A 707:373–379
Buiatti S, Boschelle O, Mozzon M, Battistutta F (1995) Determination of biogenic amines in alcoholic and non-alcoholic beers by HPLC. Food Chem 52:199–202
García-Villar N, Hernández-Cassou S, Saurina J (2009) Determination of biogenic amines in wines by pre-column derivatization and high-performance liquid chromatography coupled to mass spectrometry. J Chromatogr A 1216:6387–6393
García-Villar N, Saurina J, Hernández-Cassou S (2006) High-performance liquid chromatographic determination of biogenic amines in wines with an experimental design optimization procedure. Anal Chim Acta 575:97–105
Vidal-Carou MC, Lahoz-Portolés F, Bover-Cid S, Mariné-Font A (2003) Ion-pair high-performance liquid chromatographic determination of biogenic amines and polyamines in wine and other alcoholic beverages. J Chromatogr A 998:235–241
Hlabangana L, Hernández-Cassou S, Saurina (2006) Determination of biogenic amines in wines by ion-pair liquid chromatography and post-column derivatization with1,2-naphthoquinone-4-sulphonate, J. J Chromatogr A 1130:130–136
De Borba BM, Rohrer JS (2007) Determination of biogenic amines in alcoholic beverages by ion chromatography with suppressed conductivity detection and integrated pulsed amperometric detection. J Chromatogr A 1155:22–30
Jayarajah CN, Skelley AM, Fortner AD, Mathies RA (2007) Analysis of neuroactive amines in fermented beverages using a portable microchip capillary electrophoresis system. Anal Chem 79:8162–8169
García-Villar N, Saurina J, Hernández-Cassou S (2006) Capillary electrophoresis determination of biogenic amines by field-amplified sample stacking and in-capillary derivatization. Electrophoresis 27:474–483
Male KB, Luong JHT (2001) Derivatization, stabilization and detection of biogenic amines by cyclodextrin-modified capillary electrophoresis–laser-induced fluorescence detection. J Chromatogr A 926:309–317
Kovács A, Simon-Sarkadi L, Ganzler K (1999) Determination of biogenic amines by capillary electrophoresis. J Chromatogr A 836:305–313
Li J-S, Wang H, Huang K-J, Zhang H-S (2006) Determination of biogenic amines in apples and wine with 8-phenyl-(4-oxy-acetic acid N-hydroxysuccinimide ester)-4, 4-difluoro-1,3,5,7-tetramethyl-4-bora-3a,4a-diaza-s-indacene by high performance liquid chromatography. Anal Chim Acta 575:255–261
Cortacero-Ramírez S, Arráez-Román D, Segura-Carretero A, Fernández-Gutiérrez A (2007) Determination of biogenic amines in beers and brewing-process samples by capillary electrophoresis coupled to laser-induced fluorescence detection. Food Chem 100:383–389
Zhang N, Wang H, Zhang Z-X, Deng Y-H, Zhang H-S (2008) Sensitive determination of biogenic amines by capillary electrophoresis with a new fluorogenic reagent 3-(4-fluorobenzoyl)-2-quinolinecarboxaldehyde. Talanta 76:791–797
Huang K-J, Wei C-Y, Liu W-L, Xie W-Z, Zhang J-F, Wang W (2009) Ultrasound-assisted dispersive liquid–liquid microextraction combined with high-performance liquid chromatography-fluorescence detection for sensitive determination of biogenic amines in rice wine samples. J Chromatogr A 1216:6636–6641
Soufleros EH, Bouloumpasi E, Zotou A, Loukou Z (2007) Determination of biogenic amines in Greek wines by HPLC and ultraviolet detection after dansylation and examination of factors affecting their presence and concentration. Food Chem 101:704–716
Arce L, Ríos A, Valcárcel M (1998) Direct determination of biogenic amines in wine by integrating continuous flow clean-up and capillary electrophoresis with indirect UV detection. J Chromatogr A 803:249–260
Mazzucco E, Gosetti F, Bobba M, Marengo E, Robotti E, Gennaro MC (2010) High-performance liquid chromatography-ultraviolet detection method for the simultaneous determination of typical biogenic amines and precursor amino acids. applications in food chemistry. J Agric Food Chem 58:127–134
Santos B, Simonet BM, Ríos A, Valcárcel M (2004) Direct automatic determination of biogenic amines in wine by flow injection-capillary electrophoresis-mass spectrometry. Electrophoresis 25:3427–3433
Simó C, Moreno-Arribas MV, Cifuentes A (2008) Ion-trap versus time-of-flight mass spectrometry coupled to capillary electrophoresis to analyze biogenic amines in wine. J Chromatogr A 1195:150–156
Niculescu M, Frébort I, Peč P, Galuszka P, Mattiasson B, Csöregi E (2000) Amine oxidase based amperometric biosensors for histamine detection. Electroanalysis 12:369–375
Wimmerová M, Macholán L (1999) Sensitive amperometric biosensor for the determination of biogenic and synthetic amines using pea seedlings amine oxidase: a novel approach for enzyme immobilisation. Biosens Bioelectron 14:695–702
Bouvrette P, Male KB, Luong JHT, Gibbs BF (1997) Amperometric biosensor for diamine using diamine oxidase purified from porcine kidney. Enzyme Microb Technol 20:32–38
Male KB, Bouvrette P, Luong JHT, Gibbs BF (1996) Amperometric biosensor for total histamine, putrescine and cadaverine using diamine oxidase. J Food Sci 61:1012–1016
Keow CM, Bakar FA, Salleh AB, Heng LY, Wagiran R, Bean LS (2007) An amperometric biosensor for the rapid assessment of histamine level in tiger prawn (Penaeus monodon) spoilage. Food Chem 105:1636–1641
Carelli D, Centonze D, Palermo C, Quinto M, Rotunno T (2007) An interference free amperometric biosensor for the detection of biogenic amines in food products. Biosens Bioelectron 23:640–647
Schwelberger HG, Bodner E (1997) Purification and characterization of diamine oxidase from porcine kidney and intestine. Biochim Biophys Acta 1340:152–164
Draisci R, Volpe G, Lucentini L, Cecilia A, Federico R, Palleschi G (1998) Determination of biogenic amines with an electrochemical biosensor and its application to salted anchovies. Food Chem 62:225–232
Tombelli S, Mascini M (1998) Electrochemical biosensors for biogenic amines: a comparison between different approaches. Anal Chim Acta 358:277–284
Compagnone D, Isoldi G, Moscone D, Palleschi G (2001) Amperometric detection of biogenic amines in cheese using immobilised diamine oxidase. Anal Lett 34:841–854
Alonso-Lomillo MA, Domínguez-Renedo O, Matos P, Arcos-Martínez MJ (2010) Disposable biosensors for determination of biogenic amines. Anal Chim Acta 665:26–31
Frasconi M, Favero G, Di Fusco M, Mazzei F (2009) Polyazetidine-based immobilization of redox proteins for electron transfer based biosensors. Biosens Bioelectron 24:1424–1430
Mazzei F, Botrè F, Montilla S, Pilloton R, Podestà E, Botrè C (2004) Alkaline phosphatase inhibition based electrochemical sensors for the detection of pesticides. J Electroanal Chem 574:95–100
Di Fusco M, Tortolini C, Deriu D, Mazzei F (2010) Laccase-based biosensor for the determination of polyphenol index in wine. Talanta 81:235–240
Tortolini C, Di Fusco M, Frasconi M, Favero G, Mazzei F (2010) Laccase–polyazetidine prepolymer–MWCNT integrated system: Biochemical properties and application to analytical determinations in real samples. Microchem J 96:301–307
Paik MJ, Lee S, Cho KH, Kim KR (2006) Urinary polyamines and N-acetylated polyamines in four patients with Alzheimer's disease as their N-ethoxycarbonyl-N-pentafluoropropionyl derivatives by gas chromatography–mass spectrometry in selected ion monitoring mode. Anal Chim Acta 576:55–60
Pietrangeli P, Federico R, Mondovì B, Morpurgo L (2007) Substrate specificity of copper-containing plant amine oxidases. J Inorg Biochem 101:997–1004
Borisov IA, Lobanov AV, Reshetilov AN, Kurganov BI (2000) Quantitative analysis of the calibration dependences for biosensors. Appl Biochem Microbiol 36(3):215–220
delle Noci S, Frasconi M, Favero G, Tosi M, Ferri T, Mazzei F (2008) Electrochemical kinetic characterization of redox mediated glucose oxidase reactions: a simplified approach. Electroanalysis 2:163–169
Carelli D, Centonze D, De Giglio A, Quinto M, Zambonin PG (2006) An interference-free first generation alcohol biosensor based on a gold electrode modified by an overoxidised non-conducting polypyrrole film. Anal Chim Acta 565:27–35
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Di Fusco, M., Federico, R., Boffi, A. et al. Characterization and application of a diamine oxidase from Lathyrus sativus as component of an electrochemical biosensor for the determination of biogenic amines in wine and beer. Anal Bioanal Chem 401, 707–716 (2011). https://doi.org/10.1007/s00216-011-5131-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5131-z