Abstract
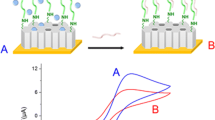
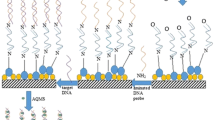
A simple and sensitive approach for the detection of marker protein, phosphinothricin acetyltransferase, from genetically modified crops was developed based on the colorimetric transition of polydiacetylene (PDA) vesicles in combination with silica microbeads. PDAs have attracted a great deal of interests as a transducing material due to their special features that allow colorimetric response to sensory signals, as well as their inherent simplicity. However, most PDA-based biosensors require additional analytical equipment such as a fluorescence microscope or UV–Vis spectrometer. In this study, we report a new approach to increase the degree of color transition by coupling antibody-conjugated PDA vesicles with silica microbeads in an effort to monitor the results with the unaided eye or simple RGB analysis. By immobilizing PDA vesicles on silica microbeads, we were able to overcome the disadvantages of colloidal PDA-based sensors and increase the degree of colorimetric changes in response to target molecules to a concentration as low as 20 nM. The additional stresses were given to PDA vesicles by antigen–antibody bridging of PDA vesicles coupled with microbeads, resulting in enhanced blue–red color transition. All the results showed that PDA vesicles in conjunction with silica microbeads will be a promising transducing material for the detection of target proteins in diagnostic and biosensing applications.







Similar content being viewed by others
Abbreviations
- DMPC:
-
1,2-Dimyristoyl phosphatidyl choline
- EDC:
-
1-Ethyl-3-(3-dimethyl amino-propyl)carbodiimide
- GMO:
-
Genetically modified organisms
- NHS:
-
N-hydroxysuccinimide
- PAT:
-
Phosphinothricin acetyltransferase
- PDA:
-
Polydiacetylene
- TCDA:
-
10,12-Tricosadiynoic acid
References
Wegner G (1969) Topochemical reactions of monomers with conjugated triple bonds I. Polymerization of derivatives of 2,4-hexadiyne-1,6-diols in the crystalline state. A Naturforschung Teil B 24(7):824–832
Tieke B (1985) Polymerization of butadiene and butadiyne (diacetylene) derivatives in layer structures. Adv Polym Sci 71:79–151
Jelinek R, Kolusheva S (2007) Biomolecular sensing with colorimetric vesicles. Top Curr Chem 277:155–180
Y-l Su, J-r L, Jiang L, Cao J (2005) Biosensor signal amplification of vesicles functionalized with glycolipid for colorimetric detection of Escherichia coli. J Colloid Interface Sci 284(1):114–119
Silbert L, Ben Shlush I, Israel E, Porgador A, Kolusheva S, Jelinek R (2006) Rapid chromatic detection of bacteria by use of a new biomimetic polymer sensor. Appl Environ Microbiol 72(11):7339–7344
Ma Z, Li J, Liu M, Cao J, Zou Z, Tu J, Jiang L (1998) Colorimetric detection of Escherichia coli by polydiacetylene vesicles functionalized with glycolipid. J Am Chem Soc 120(48):12678–12679
Reichert A, Nagy JO, Spevak W, Charych D (1995) Polydiacetylene liposomes functionalized with sialic acid bind and colorimetrically detect influenza virus. J Am Chem Soc 117(2):829–830
Charych D, Cheng Q, Reichert A, Kuziemko G, Stroh M, Nagy JO, Spevak W, Stevens RC (1996) A ‘litmus test’ for molecular recognition using artificial membranes. Chem Biol 3(2):113–120
Kolusheva S, Molt O, Herm M, Schrader T, Jelinek R (2005) Selective detection of catecholamines by synthetic receptors embedded in chromatic polydiacetylene vesicles. J Am Chem Soc 127(28):10000–10001
Cheng Q, Stevens RC (1997) Coupling of an induced fit enzyme to polydiacetylene thin films: colorimetric detection of glucose. Adv Mater 9(6):481–483
Rangin M, Basu A (2004) Lipopolysaccharide identification with functionalized polydiacetylene liposome sensors. J Am Chem Soc 126(16):5038–5039
Stanish I, Santos JP, Singh A (2001) One-step, chemisorbed immobilization of highly stable, polydiacetylenic hospholipid vesicles onto gold films. J Am Chem Soc 123(5):1008–1009
Kim J-M, Ji E-K, Woo SM, Lee H, Ahn DJ (2003) Immobilized polydiacetylene vesicles on solid substrates for use as chemosensors. Adv Mater 15(13):1118–1121
Shim HY, Lee SH, Ahn DJ, Ahn KD, Kim JM (2004) Micropatterning of diacetylenic liposomes on glass surfaces. Mater Sci Eng C 24(1–2):157–161
Kim JM, Lee YB, Yang DH, Lee JS, Lee GS, Ahn DJ (2005) A polydiacetylene-based fluorescent sensor chip. J Am Chem Soc 127(50):17580–17581
Reppy MA, Pindzola BA (2006) Polydiacetylene liposomes attached to glass fibers for fluorescent bioassays. Mater Res Soc Symp Proc 942:W13_10
Just RE, Alston JM, Zilberman D, Carter C, Gruère G (2006) International approval and labeling regulations of genetically modified food in major trading countries. In: Zilberman D (ed) Regulating agricultural biotechnology: economics and policy, vol 30, Natural resource management and policy. Springer, New York, pp 459–480
Michelini E, Simoni P, Cevenini L, Mezzanotte L, Roda A (2008) New trends in bioanalytical tools for the detection of genetically modified organisms: an update. Anal Bioanal Chem 392(3):355–367
Herouet C, Esdaile DJ, Mallyon BA, Debruyne E, Schulz A, Currier T, Hendrickx K, van der Klis R-J, Rouan D (2005) Safety evaluation of the phosphinothricin acetyltransferase proteins encoded by the pat and bar sequences that confer tolerance to glufosinate-ammonium herbicide in transgenic plants. Regul Toxicol Pharmacol 41(2):134–149
Reuter T, Aulrich K, Berk A, Flachowsky G (2002) Investigations on genetically modified maize (Bt-maize) in pig nutrition: chemical composition and nutritional evaluation. Arch Anim Nutr 56(1):23–31
Y-l Su, J-r L, Jiang L (2004) Chromatic immunoassay based on polydiacetylene vesicles. Colloids Surf, B 38(1–2):29–33
Kim K-W, Choi H, Lee GS, Ahn DJ, Oh M-K (2006) Micro-patterned polydiacetylene vesicle chips for detecting protein-protein interactions. Macromol Res 14:483–485
Reppy MA, Pindzola BA (2007) Biosensing with polydiacetylene materials: structures, optical properties and applications. Chem Commun (42):4317–4338
Kim K-W, Choi H, Lee GS, Ahn DJ, Oh M-K (2008) Effect of phospholipid insertion on arrayed polydiacetylene biosensors. Colloids Surf, B 66(2):213–217
Acevedo CA, Skurtys O, Young ME, Enrione J, Pedreschi F, Osorio F (2009) A non-destructive digital imaging method to predict immobilized yeast-biomass. LWT Food Sci Technol 42(8):1444–1449
Vermette P, Taylor S, Dunstan D, Meagher L (2001) Control over PEGylated-liposome aggregation by NeutrAvidin-Biotin interactions investigated by photon correlation spectroscopy. Langmuir 18(2):505–511
Jung YK, Kim TW, Park HG, Soh HT (2010) Specific colorimetric detection of proteins using bidentate aptamer-conjugated polydiacetylene (PDA) liposomes. Adv Funct Mater 20(18):3092–3097
Shim Y-Y, Shin W-S, Moon G-S, Kim K-H (2007) Quantitative analysis of phosphinothricin-n-acetyltransferase in genetically modified herbicide tolerant pepper by an enzyme-linked immunosorbent assay. J Microbiol Biotechnol 17(4):681–684
Yates K (ed) (1999) Detection methods for novel foods derived from genetically modified organisms. ILSI Europe, Brussels
Acknowledgement
This work was supported by a grant (Grant No. 20080401034032) from BioGreen 21 program, Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, MC., Shin, YJ., Jeon, TJ. et al. Microbead-assisted PDA sensor for the detection of genetically modified organisms. Anal Bioanal Chem 400, 777–785 (2011). https://doi.org/10.1007/s00216-011-4832-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-4832-7