Abstract
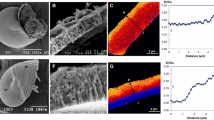
Since 1987, the Manila clam Ruditapes philippinarum has been regularly affected by the brown ring disease (BRD), an epizootic caused by the bacterium Vibrio tapetis. This disease is characterized by the development of a brown deposit on the inner face of valves. While most of the clams die from the BRD infection, some of them are able to recover by mineralizing a new repair shell layer, which covers the brown deposit by a process of encapsulation. The purpose of this work was to study the organic matrix of the shells of Manila clams in the inner shell layer before, during and after the brown deposit and during the shell repair process by confocal Raman micro-spectrometry and wavelength dispersive spectrometry (WDS) microprobe. In addition, the organic matrix of the repaired shell layer was extracted and quantified, by using standard biochemical shell matrix extractions protocols. The brown deposit exhibited high luminescence intensity in Raman spectra, and an increase of S, C, Sr (forming two peaks) and a decrease of Ca, Na concentrations (% w/w), using WDS microprobe mapping and cross-sectional transects. The signature of these trace elements was similar to that recorded on periostracal lamina (% w/w). The high S concentration likely corresponds to the presence of a high amount of sulfated organic compounds. Interestingly, on cross-sectional transects, before the brown deposit, a thin layer of the shell showed also a high luminescence, which may suggest that this layer is modified by bacteria. After the brown deposit, at the beginning of the shell repair process, the luminescence and the S concentration remain high, before declining the level found in non-BRD-affected shells. Quantification of the organic matrix shows that the shell repair layer zone is significantly different from non-BRD-affected shell layer, in particular with a much higher amount of insoluble matrix.








Similar content being viewed by others
References
Nudelman F, Gotliv BA, Addadi L, Weiner S (2006) J Struct Biol 153:176–187
Addadi L, Moradian J, Shay E, Maroudas NG, Weiner S (1987) Proc Natl Acad Sci USA 84:2732–2736
Treccani L, Mann K, Heinemann F, Fritz M (2006) Biophys J 91:2601–2608
Guzman N, Ball A, Cuif JP, Dauphin Y, Denis A, Ortlieb L (2007) Microsc Microanal 13:1–7
Marie B, Luquet G, Pais De Barros JP, Guichard N, Morel S, Alcaraz G, Bollache L, Marin F (2007) Febs J 274:2933–2945
Dauphin Y, Cuif JP, Doucet J, Salome M, Susini J, Willams CT (2003) J Struct Biol 142:272–280
Marin F, Luquet G, Marie B, Medakovic D (2008) Curr Top Dev Biol 80:209–276
Wada K (1980) Initiation of mineralization in bivalve molluscs. In: Omori M and Watabe N (eds) The mechanisms of mineralization in animals and plants, proceedings of the Third International Biomineralization Symposium. Tokai University Press, Tokai
Simkiss K (1965) Comp Biochem Physiol 16:427–435
Miyashita T, Miyamoto H and Matsushiro A (2003) Expression of pearlin in Escherichia coli, a protein which participates in the nacreous layer formation of P. fucata pearls. In: Kobayashi I and Ozawa H (eds) Biomineralization (biom 2001), formation, diversity, evolution and application, Proceedings of the 8th symposium on biomineralizations. Tokai University Press, Kanagawa, 176-177
Shen X, Belcher AM, Hansma PK, Stucky GD, Morse DE (1997) J Biol Chem 272:32472–32481
Cuif JP, Dauphin Y, Flamand D, Frerotte B, Gautret P (1986) C R Acad Sc Paris 303(série II):251–256
Cheng TC (1967) Adv Mar Biol 5:60–80
George CD (1966) Aust Gemmol 8:10–26
Paillard C, Maes P (1994) Dis Aquat Org 19:137–146
Breber P (1985) Oebelia 11:153–159
Flassch JP, Leborgne Y (1992) Introduction in Europe, from 1972 to 1980, of the Japanese Manila clam (Tapes philippinarum) and effects on aquaculture production and natural settlement. ICES Marine Symposium 194, Halifax, 92-96
Borrego JJ, Castro D, Luque A, Paillard C, Maes P, Garcia MT, Ventosa A (1996) Int J Syst Bacteriol B 46:480–484
Paillard C, Maes P (1990) C R Acad Sci Paris, Série III 310:15–20
Paillard C (2004) Aquat Living Resour 17:467–475
Paillard C, Maes P (1995) J Invertebr Pathol 65:91–100
Paillard C, Maes P (1995) J Invertebr Pathol 65:101–110
Trinkler N, Sinquin G, Querne J, Paillard C (2009) J Invertebr Pathol Submit
Jolivet A, Bardeau JF, Fablet R, Paulet YM, De Pontual H (2008) Anal Bioanal Chem 392:551–560
Jacobs DE, Soldati AL, Wirth R, Huth J, Wehrmeister U, Hofmeister W (2008) Geochim Cosmochim Acta 72:5401–5415
Takeuchi T, Sarashina I, Iijima M, Endo K (2008) FEBS Lett 582:591–596
Hedegaard C, Bardeau JF, Chateigner D (2006) Journal of Mulluscan studies 72:157–162
Yan Z, Jing G, Gong N, Li C, Zhou Y, Xie L, Zhang R (2007) Biomacromolecules 8:3597–3601
Jing G, Yan Z, Li Y, Xie L, Zhang R (2007) Mar Biotechnol 9:650–659
Urmos J, Sharma SK, Mackenzie FT (1991) Am Mineral 76:641–646
Lécuyer C, Reynard B, Martineau F (2004) Chem Geol 213:293–305
Weiss IM, Tuross N, Addadi L, Weiner S (2002) J Exp Zool 293:478–491
Gotliv BA, Addadi L, Weiner S (2003) ChemBioChem 4:522–529
Withnall R, Chowdhry BZ, Silver J, Edwards HG, de Oliveira LF (2003) Spectrochim Acta Part A 59:2207–2212
Rousseau M, Lopez E, Couté A, Mascarel G, Smith DC, Naslain R, Bourrat X (2005) J Struct Biol 149:149–157
Brink DJ, Van Der Berg NG (2005) J Phys D: Appl Phys 38:338–343
Zhang G, Xie X, Wang Y (2001) Guang Pu Xue Yu Guang Pu Fen Xi 21:193–196
Takesue RK, Bacon CR, Thompson JK (2008) Geochim Cosmochim Acta 72:5431–5445
Klein RT, Lohmann KC, Thayer CW (1996) Geochim Cosmochim Acta 60:4207–4221
Stecher HA III, Krantz DE, Lord CJ III, Luther GW III, Bock KW (1996) Geochim Cosmochim Acta 60:3445–3456
Marin F, Luquet G, Marie B, Medakovic D (2008) Curr Top Dev Biol 80:209–76
Coote GE, Trompetter WJ (1995) Nucl Instr and Meth in Phys Res B 104:333–338
Kuczumow A (2004) J Alloys Compd 362:71–82
Lingh U, Mutvei H, Sunde T, Westermark T (1988) Nucl Instr and Meth in Phys Res B 30:388–392
Crenshaw MA, Ristedt H (1976) The histochemical localization of reactive groups in septa nacre from Nautilus pompilius L. In: Watabe N, Wilbur KM (eds) The mechanisms of mineralization in the invertebrates and plants. University of South Carolina Press, USA
Fujikura K, Okoshi K, Naganuma T (2003) Mar Ecol Prog Ser 257:295–301
Labonne M, Morize E, Scolanp R, Lae R, Dabas E, Bohn M (2009) Estuar Coast Shelf Sci 82:673–682
Diouf K, Panfili J, Labonne M, Aliaume C, Tomas J, Do Chi T (2006) Environ Biol Fishes 77:9–20
Gunn JS, Harrowfield IR, Proctor CH, Thresher RE (1992) J Exp Mar Biol Ecol 158:1–36
Tzeng WN, Severin KP, Wickström H (1997) Mar Ecol Prog Ser 149:73–81
Otake T, Ishii T, Ishii T, Nakahara M, Nakamura R (1997) Mar Biol 128:565–572
Otake T, Ishii T, Nakahara M, Nakamura R (1994) Mar Ecol Prog Ser 112:189–193
Markwitz A, Grambole D, Herrmann F, Trompetter WJ, Dioses T, Gauldie RW (2000) Nucl Instr and Meth in Phys Res B 168:109–116
Elfman M, Limburg KE, Kristiansson P, Svedang H, Westin L, Wickstrom H, Malmqvist K, Pallon J (2000) Nucl Instr and Meth in Phys Res B 161–163:877–881
Radtke RL, Townsend DW, Kinzie RA III, Fey D (1999) J Exp Mar Biol Ecol 238:21–27
Dauphin Y, Cuif JP, Massard P (2006) Chem Geol 231:26–37
Marin F, Smith M, Isa Y, Muyzer G, Westbroek P (1996) Proc Natl Acad Sci USA 93:1554–1559
Gaffey SJ, Bronnimann CE (1993) J Sedim Petrol 63:752–754
Capozzi V, Perna G, Gallone A, Biagi PF, Carmone P, Fratello A, Guida G, Zanna P, Cicero R (2005) J Mol Struct 744–747:717–721
Bell SEJ, Bourguignon ESO, Grady AO, Villaumie J, Dennis AC (2002) Raman Spectroscopy 14:17–20
Schachar RA, Solin SA (1975) Invest Ophthalmol 14:380–396
Witke K, Götze J, Robler R, Dietrich D, Marx G (2004) Spectrochim Acta Part A 60:2903–2912
Crenshaw MA (1972) Biomineral Res Rep 6:6–11
Marxen JC, Becker WE (1997) Comp Biochem Physiol 118:23–33
Ravindranath MH, Rajeswari Ravindranath MH (1974) Acta Histochem. 48:26–41
Goulletquer P (1989) Etude des facteurs environnementaux intervenant sur la production de la palourde japonaise d'élevage Ruditapes philippinarum. Thesis, Université de Bretagne Occidentale, Brest
Paillard C, Maes P, Oubella R (1994) Annu Rev Fish Dis 4:219–240
Paillard C, Maes P, Mazurié J, Claude S, Marhic A, Le Pennec M (1997) Epidemiological survey of the brown ring disease in clams of Atlantic coast: role of temperature in variations of prevalence. Proceedings of the VIIIe Symposium of the International Society for Veterinary Epidemiology and Economics, AEEMA, Paris, France, 14031-14033
Acknowledgments
We would like to thank Eric Dabas and André Ogor for shell preparations for the WDS microsonde, and Nathalie Guichard for shell matrix extractions and quantifications.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trinkler, N., Labonne, M., Marin, F. et al. Clam shell repair from the brown ring disease: a study of the organic matrix using Confocal Raman micro-spectrometry and WDS microprobe. Anal Bioanal Chem 396, 555–567 (2010). https://doi.org/10.1007/s00216-009-3114-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-3114-0