Abstract
The gas-phase fragmentation reactions of singly protonated aromatic amino acids, their simple peptides as well as simple models for intermolecular disulfide bonds have been examined using a commercially available hybrid linear ion trap–Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometer. Low-energy collision-induced dissociation (CID) reactions within the linear ion trap are compared with electron-induced dissociation (EID) reactions within the FT-ICR cell. Dramatic differences are observed between low-energy CID (which occurs via vibrational excitation) and EID. For example, the aromatic amino acids mainly fragment via competitive losses of NH3 and (H2O+CO) under CID conditions, while side-chain benzyl cations are major fragment ions under EID conditions. EID also appears to be superior in cleaving the S–S and S–C bonds of models of peptides containing an intermolecular disulfide bond. Systematic studies involving fragmentation as a function of electron energy reveal that the fragmentation efficiency for EID occurs at high electron energy (more than 10 eV) compared with the low-electron energy (less than 0.2 eV) typically observed for electron capture dissociation fragmentation. Finally, owing to similarities between the types of fragment ions observed under EID conditions and those previously reported in ultraviolet photodissociation experiments and the electron-ionization mass spectra, we propose that EID results in fragmentation via electronic excitation and vibrational excitation. EID may find applications in analyzing singly charged molecular ions formed by matrix-assisted laser desorption ionization.

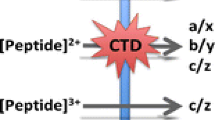
Fragmentation of singly protonated aromatic amino acids and peptide models under electron induced dissociation (EID) conditions is observed to yield complementary fragmentation to collision induced dissociation (CID), with abundant product ions are formed from bond cleavages from the sidechain








Similar content being viewed by others
Notes
The reason why these two isomeric dipeptides exhibit different behavior with regard to NH3 loss is likely to arise from the ability of the indole side chains to participate in a neighboring-group reaction. In Trp-Gly, the indole group is directly adjacent to the protonated N terminus and is thus able to participate in a neighboring-group reaction similar to protonated tryptophan (Scheme 1). In contrast, the indole side chain of Gly-Trp is further away from the protonated N terminus and isomerization of the amide bond is required for the peptide to adopt a suitable geometry for NH3 loss via a neighboring-group reaction. Other lower-energy fragmentation pathways are observed instead.
NH3 loss is absent in the CID fragmentation of protonated histidine owing to the presence of other favorable processes [38].
References
Busch KL, Glish GL, McLuckey SA (1988) Mass spectrometry/mass spectrometry. Techniques & applications of tandem mass spectrometry. VCH, New York
McLuckey SA, Goeringer DE (1997) J Mass Spectrom 32:461–474
Laskin J, Futrell JH (2003) Mass Spectrom Rev 22:158–181
Cooks RG, Jo S-C, Green J (2004) Appl Surf Sci 231–232:13–21
Laskin J (2004) Eur J Mass Spectrom 10:259–267
Grill V, Shen J, Evans C, Cooks RG (2001) Rev Sci Instrum 72:3149–3179
Fukui K, Naito Y, Akiyama Y, Takahashi K (2004) Eur J Mass Spectrom 10:639–647
Kjeldsen F, Silivra OA, Zubarev RA (2006) Chem Eur J 12:7920–7928
Marshall AG, Hendrickson CL, Jackson GS (1998) Mass Spectrom Rev 17:1–35
Laskin J, Futrell JH (2005) Mass Spectrom Rev 24:135–167
Barrow MP, Burkitt WI, Derrick PJ (2005) Analyst 130:18–28
Hakansson K, Cooper HJ, Hudgins RR, Nilsson CL (2003) Curr Org Chem 7:1503–1525
Smith RD (2000) Int J Mass Spectrom 200:509–544
Zubarev RA, Haselmann KF, Budnik BA, Kjeldsen F, Jensen F (2002) Eur J Mass Spectrom 8:337–349
Zubarev RA (2003) Mass Spectrom Rev 22:57–77
Cooper HJ, Hakansson K, Marshall AG (2005) Mass Spectrom Rev 24:201–222
Zubarev RA, Kelleher NL, McLafferty FW (1998) J Am Chem Soc 120:3265–3266
Zubarev RA, Kruger NA, Fridriksson EK, Lewis MA, Horn DM, Carpenter BK, McLafferty FW (1999) J Am Chem Soc 121:3265–3266
Kjeldsen F, Haselmann KF, Budnik BA, Jensen F, Zubarev RA (2002) Chem Phys Lett 356:201–206
Haselmann KF, Budnik BA, Kjeldsen F, Nielsen ML, Olsen JV, Zubarev RA (2002) Eur J Mass Spectrom 8:117–121
Budnik BA, Haselmann KF, Zubarev RA (2001) Chem Phys Lett 342:299–302
Adams NG, Poterya V, Babcock LM (2006) Mass Spectrom Rev 25:798–828
Budnik BA, Zubarev RA (2000) Chem Phys Lett 316:19–23
Cody RB, Freiser BS (1979) Anal Chem 51:547–551
Cody RB, Freiser BS (1987) Anal Chem 59:1054–1056
Gord JR, Horning SR, Wood JM, Cooks RG, Freiser BS (1993) J Am Soc Mass Spectrom 4:145–151
Nielsen ML, Budnik BA, Haselmann KF, Olsen JV, Zubarev RA (2000) Chem Phys Lett 330:558–562
Nielsen ML, Budnik BA, Haselmann KF, Zubarev RA (2003) Int J Mass Spectrom 226:181–187
Khairallah GN, O’Hair RAJ, Bruce MI (2006) Dalton Trans 3699–3707
Malek R, Metelmann-Strupat W, Zeller M, Muenster H (2005) Am Biotech Lab 8–9
Lioe H, O’Hair RAJ, Reid GE (2004) J Am Soc Mass Spectrom 15:65–76
Kang H, Dedonder-Lardeux C, Jouvet C, Martrenchard S, Gregoire G, Desfrancois C, Schermann J-P, Barat M, Fayeton JA (2004) Phys Chem Chem Phys 6:2628–2632
Nolting D, Marian C, Weinkauf R (2004) Phys Chem Chem Phys 6:2633–2640
Kang H, Jouvet C, Dedonder-Lardeux C, Martrenchard S, Gregoire G, Desfrancois C, Schermann J-P, Barat M, Fayeton JA (2005) Phys Chem Chem Phys 7:394–398
Talbot FO, Tabarin T, Antoine R, Broyer M, Dugourd P (2005) J Chem Phys 122:074310/1–5
Barlow CK, Moran D, Radom L, McFadyen WD, O’Hair RAJ (2006) J Phys Chem A 110:8304–8315
Lioe H, O’Hair RAJ (2005) Org Biomol Chem 3:3618–3628
Farrugia JM, Taverner T, O’Hair RAJ (2001) Int J Mass Spectrom 209:99–112
Dookeran NN, Yalcin T, Harrison AG (1996) J Mass Spectrom 31:500–508
Rogalewicz F, Hoppilliard Y, Ohanessian G (2000) Int J Mass Spectrom 195/196:565–590
El Aribi H, Orlova G, Hopkinson AC, Siu KWM (2004) J Phys Chem A 108:3844–3853
Sorensen M, Forster JS, Hvelplund P, Jorgensen TJD, Nielsen SB, Tomita S (2001) Chem Eur J 7:3214–3222
Stein SE (2005) In: Linstrom PJ, Mallard WG (eds) NIST chemistry WebBook. NIST standard reference database number 69. National Institute of Standards and Technology, Gaithersburg http://webbook.nist.gov
Sobolewski AL, Domcke W, Dedonder-Lardeux C, Jouvet C (2002) Phys Chem Chem Phys 4:1093–1100
Rinker A, Halleman CD, Wedlock MR (2005) Chem Phys Lett 414:505–508
Barr J, Torres I, Verdasco E, Banares L, Aoiz FJ, Martinez-Haya B (2004) J Phys Chem A 108:7936–7948
Gorman JJ, Wallis TP, Pitt JJ (2002) Mass Spectrom Rev 21:183–216
Lioe H, O’Hair RAJ (2007) J Am Soc Mass Spectrom 18:1109–1123
Fung YME, Kjeldsen F, Silivra OA, Chan TWD, Zubarev RA (2005) Angew Chem Int Ed Engl 44:6399–6403
Zubarev RA, Kruger NA, Fridriksson EK, Lewis MA, Horn DM, Carpenter BK, McLafferty FW (1999) J Am Chem Soc 121:2857–2862
Savitski MM, Kjeldsen F, Nielsen ML, Zubarev RA (2006) Angew Chem Int Ed Engl 45:5301–5303
Stingl C, Ihling C, Ammerer G, Sinz A, Mechtler K (2006) Biochim Biophys Acta 1764:1842–1852
Acknowledgements
R.A.J.O. thanks the Australian Research Council for support of this work under the ARC Centres of Excellence program (ARC Centre of Excellence in Free Radical Chemistry and Biotechnology), the ARC-Lief program and VICS for helping fund the purchase of the LTQ FT FT-ICR instrument. H.L. acknowledges the award of an Elizabeth and Vernon Puzey Postgraduate Scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Part 56 of the series “Gas phase ion chemistry of biomolecules”.
Rights and permissions
About this article
Cite this article
Lioe, H., O’Hair, R.A.J. Comparison of collision-induced dissociation and electron-induced dissociation of singly protonated aromatic amino acids, cystine and related simple peptides using a hybrid linear ion trap–FT-ICR mass spectrometer. Anal Bioanal Chem 389, 1429–1437 (2007). https://doi.org/10.1007/s00216-007-1535-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-007-1535-1