Abstract
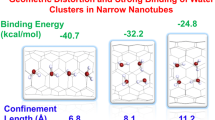
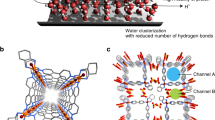
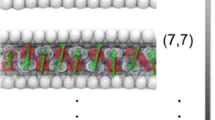
Protonated water clusters confined to the carbon nanotube (CNT) channels of sub-nanometer diameter is beyond the realm of a continuum description. The steric compulsions offered by the narrow geometry can restrict the formation of hydrogen bond between the water molecules but enhance water and channel interactions. Herein, we have made an attempt to investigate the factors, which are strongly affecting the structure and energy barrier for proton transfer in Zundel cation under the confinement of CNT by employing dispersion-corrected density functional theory-based methods. Our results reveal that the diverse nature of water–water and water–wall interaction inside the nanotube for different size of CNT channels can have remarkable effects on the energetics of the proton transfer process, geometrical parameters, oscillatory shuttling motion of the proton and various energy components, viz. interaction energy, hydrogen bond energy, etc. Due to these factors, the proton oscillation in Zundel cation is shown to be nonmonotonic in nature with respect to the degree of confinement. Finally, we have demonstrated that the effect of confinement rendered by CNT(6,6) on Zundel cation can be the best suitable candidate among the series of CNTs considered in the present study, for assisting the proton transfer in the Zundel cation easily. These conclusions can have important implications and motivate further investigations to understand the fluidics under confined nanomaterials.











Similar content being viewed by others
References
Wang JH (1968) Facilitated proton transfer in enzyme catalysis. Science 161(3839):328–334
Kreuer K-D, Paddison SJ, Spohr E, Schuster M (2004) Transport in proton conductors for fuel-cell applications: simulations, elementary reactions, and phenomenology. Chem Rev 104(10):4637–4678. doi:10.1021/cr020715f
Rasaiah JC, Garde S, Hummer G (2008) Water in nonpolar confinement: from nanotubes to proteins and beyond. Annu Rev Phys Chem 59(1):713–740. doi:10.1146/annurev.physchem.59.032607.093815
Balbuena P, Seminario J (eds) (2006) Nanomaterials: design and simulation. Theoretical and computational chemistry, 1st edn. Elsevier, Amsterdam
Mancinelli R (2010) The effect of confinement on water structure. J Phys Condens Matter 22(40):404213
Granick S (1991) Motions and relaxations of confined liquids. Science 253(5026):1374–1379. doi:10.1126/science.253.5026.1374
Thompson WH (2011) Solvation dynamics and proton transfer in nanoconfined liquids. Annu Rev Phys Chem 62(1):599–619. doi:10.1146/annurev-physchem-032210-103330
Hummer G, Rasaiah JC, Noworyta JP (2001) Water conduction through the hydrophobic channel of a carbon nanotube. Nature 414(6860):188–190
Noy A, Park HG, Fornasiero F, Holt JK, Grigoropoulos CP, Bakajin O (2007) Nanofluidics in carbon nanotubes. Nano Today 2(6):22–29. doi:10.1016/S1748-0132(07)70170-6
Jiao Y, Du A, Hankel M, Smith SC (2013) Modelling carbon membranes for gas and isotope separation. Phys Chem Chem Phys 15(14):4832–4843. doi:10.1039/c3cp44414g
Alexiadis A, Kassinos S (2008) Molecular simulation of water in carbon nanotubes. Chem Rev 108(12):5014–5034. doi:10.1021/cr078140f
Kofinger J, Hummer G, Dellago C (2011) Single-file water in nanopores. Phys Chem Chem Phys 13(34):15403–15417. doi:10.1039/c1cp21086f
Mukherjee B, Maiti PK, Dasgupta C, Sood AK (2007) Strong correlations and Fickian water diffusion in narrow carbon nanotubes. J Chem Phys 126 (12). doi:10.1063/1.2565806
Liu Y, Wang Q, Wu T, Zhang L (2005) Fluid structure and transport properties of water inside carbon nanotubes. J Chem Phys 123 (23). doi:10.1063/1.2131070
Baughman RH, Zakhidov AA, de Heer WA (2002) Carbon nanotubes—the route toward applications. Science 297(5582):787–792. doi:10.1126/science.1060928
Corry B (2008) Designing carbon nanotube membranes for efficient water desalination. J Phys Chem B 112(5):1427–1434. doi:10.1021/jp709845u
Ulissi ZW, Shimizu S, Lee CY, Strano MS (2011) Carbon nanotubes as molecular conduits: advances and challenges for transport through isolated sub-2 nm pores. J Phys Chem Lett 2(22):2892–2896. doi:10.1021/jz201136c
Ghosh S, Sood AK, Kumar N (2003) Carbon nanotube flow sensors. Science 299(5609):1042–1044. doi:10.1126/science.1079080
Zuo G, Shen R, Ma S, Guo W (2010) Transport properties of single-file water molecules inside a carbon nanotube biomimicking water channel. ACS Nano 4(1):205–210. doi:10.1021/nn901334w
Wang H-J, Xi X-K, Kleinhammes A, Wu Y (2008) Temperature-induced hydrophobic–hydrophilic transition observed by water adsorption. Science 322(5898):80–83. doi:10.1126/science.1162412
Kolesnikov AI, Zanotti J-M, Loong C-K, Thiyagarajan P, Moravsky AP, Loutfy RO, Burnham CJ (2004) Anomalously soft dynamics of water in a nanotube: a revelation of nanoscale confinement. Phys Rev Lett 93(3):035503
Byl O, Liu J-C, Wang Y, Yim W-L, Johnson JK, Yates JT (2006) Unusual hydrogen bonding in water-filled carbon nanotubes. J Am Chem Soc 128(37):12090–12097. doi:10.1021/ja057856u
Cambré S, Schoeters B, Luyckx S, Goovaerts E, Wenseleers W (2010) Experimental observation of single-file water filling of thin single-wall carbon nanotubes down to chiral index (5,3). Phys Rev Lett 104(20):207401
Naguib N, Ye H, Gogotsi Y, Yazicioglu AG, Megaridis CM, Yoshimura M (2004) Observation of water confined in nanometer channels of closed carbon nanotubes. Nano Lett 4(11):2237–2243. doi:10.1021/nl0484907
Gordillo MC, Martı́ J (2000) Hydrogen bond structure of liquid water confined in nanotubes. Chem Phys Lett 329(5–6):341–345. doi:10.1016/S0009-2614(00)01032-0
Striolo A (2006) The mechanism of water diffusion in narrow carbon nanotubes. Nano Lett 6(4):633–639
Joseph S, Aluru NR (2008) Why are carbon nanotubes fast transporters of water? Nano Lett 8(2):452–458
Mashl RJ, Joseph S, Aluru NR, Jakobsson E (2003) Anomalously immobilized water: a new water phase induced by confinement in nanotubes. Nano Lett 3(5):589–592. doi:10.1021/nl0340226
Koga K, Gao GT, Tanaka H, Zeng XC (2001) Formation of ordered ice nanotubes inside carbon nanotubes. Nature 412(6849):802–805
Cao Z, Peng Y, Yan T, Li S, Li A, Voth GA (2010) Mechanism of fast proton transport along one-dimensional water chains confined in carbon nanotubes. J Am Chem Soc 132(33):11395–11397. doi:10.1021/ja1046704
Agmon N (1995) The Grotthuss mechanism. Chem Phys Lett 244(5–6):456–462. doi:10.1016/0009-2614(95)00905-j
Markovitch O, Chen H, Izvekov S, Paesani F, Voth GA, Agmon N (2008) Special pair dance and partner selection: elementary steps in proton transport in liquid water. J Phys Chem B 112(31):9456–9466. doi:10.1021/jp804018y
Liang C, Jansen TLC, Knoester J (2011) Proton transport in biological systems can be probed by two-dimensional infrared spectroscopy. J Chem Phys 134(4):044502–044508
Garczarek F, Gerwert K (2006) Functional waters in intraprotein proton transfer monitored by FTIR difference spectroscopy. Nature 439(7072):109–112
Freier E, Wolf S, Gerwert K (2011) Proton transfer via a transient linear water-molecule chain in a membrane protein. Proc Natl Acad Sci 108(28):11435–11439. doi:10.1073/pnas.1104735108
Dellago C, Naor MM, Hummer G (2003) Proton transport through water-filled carbon nanotubes. Phys Rev Lett 90(10):105902
Bankura A, Chandra A (2012) Hydroxide ion can move faster than an excess proton through one-dimensional water chains in hydrophobic narrow pores. J Phys Chem B 116(32):9744–9757. doi:10.1021/jp301466e
Mann DJ, Halls MD (2003) Water alignment and proton conduction inside carbon nanotubes. Phys Rev Lett 90(19):195503
Habenicht BF, Paddison SJ, Tuckerman ME (2010) Ab initio molecular dynamics simulations investigating proton transfer in perfluorosulfonic acid functionalized carbon nanotubes. Phys Chem Chem Phys 12(31):8728–8732
Habenicht BF, Paddison SJ, Tuckerman ME (2010) The effects of the hydrophobic environment on proton mobility in perfluorosulfonic acid systems: an ab initio molecular dynamics study. J Mater Chem 20(30):6342–6351. doi:10.1039/c0jm00253d
Brewer ML, Schmitt UW, Voth GA (2001) The formation and dynamics of proton wires in channel environments. Biophys J 80(4):1691–1702. doi:10.1016/S0006-3495(01)76140-1
Chen J, Li X-Z, Zhang Q, Michaelides A, Wang E (2013) Nature of proton transport in a water-filled carbon nanotube and in liquid water. Phys Chem Chem Phys 15(17):6344–6349. doi:10.1039/c3cp50218j
Scheiner S (1981) Proton transfers in hydrogen-bonded systems. Cationic oligomers of water. J Am Chem Soc 103(2):315–320. doi:10.1021/ja00392a012
Ojamäe L, Shavitt I, Singer SJ (1995) Potential energy surfaces and vibrational spectra of H5O2+ and larger hydrated proton complexes. Int J Quantum Chem 56(S29):657–668. doi:10.1002/qua.560560872
Barone V, Orlandini L, Adamo C (1995) Proton transfer in small model systems: a density functional study. Int J Quantum Chem 56(6):697–705. doi:10.1002/qua.560560607
Lao-ngam C, Asawakun P, Wannarat S, Sagarik K (2011) Proton transfer reactions and dynamics in protonated water clusters. Phys Chem Chem Phys 13(10):4562–4575
Lao-ngam C, Phonyiem M, Chaiwongwattana S, Kawazoe Y, Sagarik K (2013) Characteristic NMR spectra of proton transfer in protonated water clusters. Chem Phys 420:50–61. doi:10.1016/j.chemphys.2013.04.017
Headrick JM, Diken EG, Walters RS, Hammer NI, Christie RA, Cui J, Myshakin EM, Duncan MA, Johnson MA, Jordan KD (2005) Spectral signatures of hydrated proton vibrations in water clusters. Science 308(5729):1765–1769. doi:10.1126/science.1113094
Shin I, Park M, Min SK, Lee EC, Suh SB, Kim KS (2006) Structure and spectral features of H[sup+](H[sub 2]O)[sub 7]: Eigen versus Zundel forms. J Chem Phys 125(23):234305–234307
Parthasarathi R, Subramanian V, Sathyamurthy N (2007) Hydrogen bonding in protonated water clusters: an atoms-in-molecules perspective. J Phys Chem A 111(51):13287–13290. doi:10.1021/jp0775909
Treutler O, Ahlrichs R (1995) Efficient molecular numerical integration schemes. J Chem Phys 102(1):346–354
Ahlrichs R, Bär M, Häser M, Horn H, Kölmel C (1989) Electronic structure calculations on workstation computers: the program system turbomole. Chem Phys Lett 162(3):165–169. doi:10.1016/0009-2614(89)85118-8
Xie Y, Remington RB, Schaefer Iii HF (1994) The protonated water dimer: extensive theoretical studies of H[sub 5]O[sup +][sub 2]. J Chem Phys 101(6):4878–4884
Geissler PL, Van Voorhis T, Dellago C (2000) Potential energy landscape for proton transfer in (H2O)3H+: comparison of density functional theory and wavefunction-based methods. Chem Phys Lett 324(1–3):149–155. doi:10.1016/s0009-2614(00)00479-6
Bryantsev VS, Diallo MS, van Duin ACT, Goddard WA (2009) Evaluation of B3LYP, X3LYP, and M06-class density functionals for predicting the binding energies of neutral, protonated, and deprotonated water clusters. J Chem Theory Comput 5(4):1016–1026. doi:10.1021/ct800549f
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27(15):1787–1799. doi:10.1002/jcc.20495
Lee EPF, Dyke JM (1991) An Ab Initio molecular orbital study of protonated water clusters, H(H2O) n + n = 1 to 5, at the SCF and MP2 levels. Mol Phys 73(2):375–405. doi:10.1080/00268979100101261
Valeev EF, Schaefer Iii HF (1998) The protonated water dimer: Brueckner methods remove the spurious C[sub 1] symmetry minimum. J Chem Phys 108(17):7197–7201
Auer AA, Helgaker T, Klopper W (2000) Accurate molecular geometries of the protonated water dimer. Phys Chem Chem Phys 2(10):2235–2238
Park M, Shin I, Singh NJ, Kim KS (2007) Eigen and Zundel forms of small protonated water clusters: structures and infrared spectra. J Phys Chem A 111(42):10692–10702. doi:10.1021/jp073912x
Sobolewski AL, Domcke W (2002) Ab initio investigation of the structure and spectroscopy of hydronium–water clusters. J Phys Chem A 106(16):4158–4167. doi:10.1021/jp013835k
Stoyanov ES, Stoyanova IV, Tham FS, Reed CA (2009) Haq+ structures in proton wires inside nanotubes. J Am Chem Soc 131(48):17540–17541. doi:10.1021/ja907708g
Luecke H, Schobert B, Richter H-T, Cartailler J-P, Lanyi JK (1999) Structural changes in bacteriorhodopsin during ion transport at 2 angstrom resolution. Science 286(5438):255–260
Suzuki S, Green PG, Bumgarner RE, Dasgupta S, Goddard WA, Blake GA (1992) benzene forms hydrogen bonds with water. Science 257(5072):942–945. doi:10.1126/science.257.5072.942
Rozas I, Alkorta I, Elguero J (1997) Unusual hydrogen bonds: H···π interactions. J Phys Chem A 101(49):9457–9463. doi:10.1021/jp971893t
Wang L, Zhao J, Li F, Fang H, Lu JP (2009) First-principles study of water chains encapsulated in single-walled carbon nanotube. J Phys Chem C 113(14):5368–5375. doi:10.1021/jp808873r
Furutaka S, Ikawa S-I (2002) pi-hydrogen bonding between water and aromatic hydrocarbons at high temperatures and pressures. J Chem Phys 117(2):751–755
Cicero G, Grossman JC, Schwegler E, Gygi F, Galli G (2008) Water confined in nanotubes and between graphene sheets: a first principle study. J Am Chem Soc 130(6):1871–1878. doi:10.1021/ja074418+
Yeh LI, Okumura M, Myers JD, Price JM, Lee YT (1989) Vibrational spectroscopy of the hydrated hydronium cluster ions H[sub 3]O[sup +] [center-dot] (H[sub 2]O)[sub n] (n = 1, 2, 3). J Chem Phys 91(12):7319–7330
Yeh LI, Lee YT, Hougen JT (1994) Vibration-rotation spectroscopy of the hydrated hydronium ions H5O+2 and H9O+4. J Mol Spectrosc 164(2):473–488. doi:10.1006/jmsp.1994.1090
Asmis KR, Pivonka NL, Santambrogio G, Brümmer M, Kaposta C, Neumark DM, Wöste L (2003) Gas-phase infrared spectrum of the protonated water dimer. Science 299(5611):1375–1377. doi:10.1126/science.1081634
Fridgen TD, McMahon TB, MacAleese L, Lemaire J, Maitre P (2004) Infrared spectrum of the protonated water dimer in the gas phase. J Phys Chem A 108(42):9008–9010. doi:10.1021/jp040486w
Headrick JM, Bopp JC, Johnson MA (2004) Predissociation spectroscopy of the argon-solvated H[sub 5]O[sub 2][sup +] “zundel’” cation in the 1000–1900 cm[sup - 1] region. J Chem Phys 121(23):11523–11526
Hammer NI, Diken EG, Roscioli JR, Johnson MA, Myshakin EM, Jordan KD, McCoy AB, Huang X, Bowman JM, Carter S (2005) The vibrational predissociation spectra of the H[sub 5]O[sub 2][sup +] [center-dot] RG[sub n](RG = Ar, Ne) clusters: correlation of the solvent perturbations in the free OH and shared proton transitions of the Zundel ion. J Chem Phys 122(24):244301–244310
Douberly GE, Walters RS, Cui J, Jordan KD, Duncan MA (2010) infrared spectroscopy of small protonated water clusters, H + (H2O)n (n = 2 − 5): isomers, argon tagging, and deuteration. J Phys Chem A 114(13):4570–4579. doi:10.1021/jp100778s
Agostini F, Vuilleumier R, Ciccotti G (2011) Infrared spectroscopy and effective modes analysis of the protonated water dimer H[sup +](H[sub 2]O)[sub 2] at room temperature under H/D substitution. J Chem Phys 134(8):084303–084310
Vendrell O, Gatti F, Meyer H-D (2007) Dynamics and infrared spectroscopy of the protonated water dimer. Angew Chem Int Ed 46(36):6918–6921. doi:10.1002/anie.200702201
Sauer J, Döbler J (2005) Gas-phase infrared spectrum of the protonated water dimer: molecular dynamics simulation and accuracy of the potential energy surface. ChemPhysChem 6(9):1706–1710. doi:10.1002/cphc.200500075
Jena N, Tripathy M, Samanta A, Chandrakumar KRS, Ghosh S (2012) Water molecule encapsulated in carbon nanotube model systems: effect of confinement and curvature. Theor Chem Acc 131(4):1–12. doi:10.1007/s00214-012-1205-z
Chandrakumar KRS, Srinivasu K, Ghosh SK (2008) Nanoscale curvature-induced hydrogen adsorption in alkali metal doped carbon nanomaterials. J Phys Chem C 112(40):15670–15679. doi:10.1021/jp8019446
Wang Z, Irle S, Zheng G, Morokuma K (2008) Analysis of the relationship between reaction energies of electrophilic SWNT additions and sidewall curvature: chiral nanotubes. J Phys Chem C 112(33):12697–12705
Zheng G, Wang Z, Irle S, Morokuma K (2006) Origin of the linear relationship between CH2/NH/O-SWNT reaction energies and sidewall curvature: armchair nanotubes. J Am Chem Soc 128(47):15117–15126
Hirunsit P, Balbuena PB (2007) Effects of confinement on small water clusters structure and proton transport. J Phys Chem A 111(42):10722–10731. doi:10.1021/jp074818j
Kumar RM, Elango M, Subramanian V (2010) Carbohydrate–aromatic interactions: the role of curvature on XH···π interactions. J Phys Chem A 114(12):4313–4324
Umadevi D, Sastry GN (2012) Metal ion binding with carbon nanotubes and graphene: effect of chirality and curvature. Chem Phys Lett 549:39–43
Acknowledgments
The authors gratefully acknowledge Profs. B. N. Jagatap, R. S. Sharma and P. V. Varde for their kind support and encouragements. They also acknowledge the computer center of BARC for providing the high-performance parallel computing facility (Adhya and Ajeya). S.K.G acknowledges DST-India for Sir J. C Bose Fellowship and also support from the INDO-EU project MONAMI.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tripathy, M.K., Jena, N.K., Samanta, A.K. et al. Effect of confinement on the structure and energetics of Zundel cation present inside the hydrophobic carbon nanotubes: an ab initio study. Theor Chem Acc 133, 1576 (2014). https://doi.org/10.1007/s00214-014-1576-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-014-1576-4