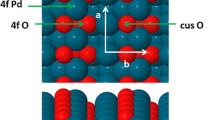
Abstract
A decomposition scheme is proposed to analyze the physical contributions to the decrease in the binding energy of chemisorbed species with increasing coverage. This scheme is applied to the acetaldehyde–TiO2 (110) rutile system as a model for other small organic molecule—oxide surface systems. Different density functional theory (DFT) functionals have been employed at both low-medium and high coverages to understand how the different theoretical descriptions of the various terms influence the adsorbate–surface interaction. At low coverages, it is found that the localized adsorbate to surface electron donation is the fundamental physical process that influences the adsorbate–surface interaction. This results shows that while it is usually assumed that only pairwise adsorbate–adsorbate interactions influence the adsorption energy, the progressive modification of the surface properties (surface reduction in this case) may also play a significative role. The DFT+U functional results, in this case, in the best agreement with the experimental binding energy, and the inclusion of the dispersive forces results in largely overestimated adsorption energies. At higher coverages, the pure GGA and GGA+U functionals overestimate the repulsive terms and the computed binding energy is well below the experimental data. The inclusion of the dispersive forces is required to correctly reproduce the experimental results. The contributions of the different physical terms are also analyzed.




Similar content being viewed by others
References
Mallat T, Baiker A (2004) Chem Rev 104:3037–3058
Hashmi ASK, Hutchings GJ (2006) Angew Chem Int Ed 45:7896–7936
Hoffmann MR, Martin ST, Choi WY, Bahnemann DW (1995) Chem Rev 95:69–96
Kubacka A, Bachiller-Baeza B, Colón G, Fernández-García M (2009) J Phys Chem C 113:8553–8555
Schreiber F (2000) Prog Surf Sci 65:151–256
Smith RK, Lewis PA, Weiss PS (2004) Prog Surf Sci 75:1–68
O’Regan B, Grätzel M (1991) Nature 353:737–740
Bach U, Lupo D, Comte P, Moser JE, Weissortel F, Salbeck J, Spreitzer H, Grätzel M (1998) Nature 395:583–585
Hagleitner C, Hierlemann A, Lange D, Kummer A, Kerness N, Brand O, Baltes H (2001) Nature 414:293–296
Li J, Lu YJ, Ye Q, Cinke M, Han J, Meyyappan M (2003) Nano Lett 3:929–933
Chorkendor I, Niemantsverdriet J (2003) Concepts of modern catalysis and kinetics. Wiley-VCH, Weinheim
Diebold U (2003) Surf Sci Rep 48:53–229
Linsebigler AL, Lu GQ, Yates JT (1995) Chem Rev 95:735–758
Thompson TL, Yates JT (2006) Chem Rev 106:4428–4453
Sauer ML, Ollis DF (1996) J Catal 158:570–582
Falconer JL, Magrini-Bair KA (1998) J Catal 179:171–178
Fujiwara N, Friedrich KA, Stimming U (1999) J Elec Chem 472:120–125
Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y (2001) Science 293:269–271
Deluga GA, Salge JR, Schmidt LD, Verykios XE (2004) Science 303:993–997
Zehr RT, Henderson MA (2008) Surf Sci 602:2238–2249
Kresse G, Furthmuller J (1996) Phys Rev B 54:11169–11186
Kresse G, Furthmuller J (1996) Comput Mater Sci 6:15–50
Kresse G, Hafner J (1993) Phys Rev B 47:558–561
Kresse G, Joubert D (1999) Phys Rev B 59:1758–1775
Blöchl PE (1994) Phys Rev B 50:17953–17979
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Phys Rev B 46:6671–6687
Harris J (1985) Phys Rev B 31:1770–1779
Henkelman G, Arnaldsson A, Jonsson H (2006) Comput Mater Sci 36:354–360
Sanville E, Kenny SD, Smith R, Henkelman G (2007) J Comput Chem 28:899–908
Bader RFW (1985) Acc Chem Res 18:9–15
Rasmussen MD, Molina ML, Hammer B (2004) J Chem Phys 120:14583
Ganduglia-Pirovano MV, Hofmann A, Sauer J (2007) Surf Sci Rep 62:219–270
Dudarev SL, Botton GA, Savrasov SY, Humphreys CJ, Sutton AP (1998) Phys Rev B 57:1505–1509
Deskins NA, Rousseau R, Dupuis M (2009) J Phys Chem C 113:14583–14586
Park JB, Graciani J, Evans J, Stacchiola D, Ma SG, Liu P, Nambu A, Sanz JF, Hrbek J, Rodriguez JA (2009) Proc Natl Acad Sci 106:4975–4980
Dion M, Rydberg H, Schrder E, Langreth DC, Lundqvist BI (2004) Phys Rev Lett 92:246401
Klimeš J, Michaelides A (2012) J Chem Phys 137:120901
Román-Pérez G, Soler JM (2009) Phys Rev Lett 103:096102
Klimeš J, Bowler DR, Michaelides A (2011) Phys Rev B 83:195131
Oviedo J, Miguel MAS, Sanz JF (2004) J Chem Phys 121:7427–7433
Márquez AM, Plata JJ, Sanz JF (2009) J Phys Chem C 113:19973–19980
Henderson MA (2004) J Phys Chem B 108:18932–18941
Plata JJ, Collico V, Márquez AM, Sanz JF (2011) J Phys Chem C 115:2819–2825
Koch W, Holthausen MC (2002) A chemist guide to density functional theory. Wiley, Mörlenbach
Tkatchenko A, Romaner L, Hofmann OT, Zojer E, Ambrosch-Draxl C, Scheffler M (2010) MRS Bull 35:435–442
Göltl F, Hafner J (2011) J Chem Phys 134:064102
Redhead PA (1962) Vacuum 12:203
Acknowledgments
This work was funded by the Spanish Ministerio de Educación y Ciencia, MEC (project MAT2008-04918) and the Junta de Andalucía (project P08-FQM-03661). Part of the computer time was provided by the Centro Informático Científico de Andalucía (CICA). V. C. thanks Università degli Studi di Milano for a Socrates-Erasmus fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published as part of the special collection of articles derived from the 8th Congress on Electronic Structure: Principles and Applications (ESPA 2012).
Rights and permissions
About this article
Cite this article
Plata, J.J., Collico, V., Márquez, A.M. et al. Analysis of the origin of lateral interactions in the adsorption of small organic molecules on oxide surfaces. Theor Chem Acc 132, 1311 (2013). https://doi.org/10.1007/s00214-012-1311-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-012-1311-y