Abstract
Rationale
It is presently unclear whether diabetic rats experience greater rewarding effects of nicotine and/or negative affective states produced by nicotine withdrawal.
Objective
The present study utilized a rodent model of diabetes to examine the rewarding effects of nicotine and negative affective states and physical signs produced by withdrawal.
Methods
Separate groups of rats received systemic administration of either vehicle or streptozotocin (STZ), which destroys insulin-producing beta cells in the pancreas and elevates glucose levels. Place conditioning procedures were utilized to compare the rewarding effects of nicotine (conditioned place preference; CPP) and negative affective states produced by withdrawal (conditioned place aversion; CPA) in vehicle- and STZ-treated rats. CPA and physical signs of withdrawal were compared after administration of the nicotinic receptor antagonist mecamylamine to precipitate withdrawal in nicotine-dependent rats. A subsequent study utilized elevated plus maze (EPM) procedures to compare anxiety-like behavior produced by nicotine withdrawal in vehicle- and STZ-treated rats.
Results
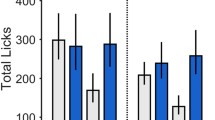
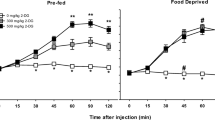
STZ-treated rats displayed greater rewarding effects of nicotine and a larger magnitude of aversive effects and physical signs produced by withdrawal as compared to vehicle-treated controls. STZ-treated rats also displayed higher levels of anxiety-like behavior on the EPM during nicotine withdrawal as compared to controls.
Conclusion
The finding that both nicotine reward and withdrawal are enhanced in a rodent model of diabetes implies that the strong behavioral effects of nicotine promote tobacco use in persons with metabolic disorders, such as diabetes.




Similar content being viewed by others
References
Balfour DJ (2002) Neuroplasticity within the mesoaccumbens dopamine system and its role in tobacco dependence. Curr Drug Targets CNS Neuro Disord 1:413–421
Bell RH Jr, Hye RJ (1983) Animal models of diabetes mellitus: physiology and pathology. J Surg Res 35:433–460
Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, et al. (2000) Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs. Suppl:i - iv, 1–112
Blum K, Chen AL, Chen TJ, Braverman ER, Reinking J, Blum SH et al (2008) Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): a commentary. Theor Biol Med Model 5:24
Fan AZ, Rock V, Zhang X, Li Y, Elam-Evans L, Balluz L (2013) Trends in cigarette smoking rates and quit attempts among adults with and without diagnosed diabetes, United States, 2001–2010. Prev Chronic Dis 10:E160
Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, Sahakian BJ, Robbins TW, Bullmore ET, Hollander E (2010) Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology 35:591–604
Fudala PJ, Teoh KW, Iwamoto ET (1985) Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol Biochem Behav 22:237–241
George DN, Jenkins TA, Killcross S (2011) Dissociation of prefrontal cortex and nucleus accumbens dopaminergic systems in conditional learning in rats. Behav Brain Res 225:47–55
Ghitza UE, Wu LT, Tai B (2013) Integrating substance abuse care with community diabetes care: implications for research and clinical practice. Subst Abuse Rehabil 4:3–10
Haire-Joshu D, Heady S, Thomas L, Schechtman K, Fisher EB Jr (1994) Depressive symptomatology and smoking among persons with diabetes. Res Nurs Health 17:273–282
Holt RI, Cockram C, Flyvbjerg A, Goldstein BJ (2010) Textbook of diabetes. 4th ed. Wiley-Blackwell
Janhunen S, Linnervuo A, Svensk M, Ahtee L (2005) Effects of nicotine and epibatidine on locomotor activity and conditioned place preference in rats. Pharmacol Biochem Behav 82:758–765
Le Foll B, Goldberg SR (2005) Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharm (Berl) 178:481–492
Natividad LA, Tejeda HA, Torres OV, O’Dell LE (2010) Nicotine withdrawal produces a decrease in extracellular levels of dopamine in the nucleus accumbens that is lower in adolescent versus adult male rats. Synapse 64:136–145
Ng RS, Darko DA, Hillson RM (2004) Street drug use among young patients with type I diabetes in the UK. Diabet Med 21:295–296
O’Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A (2006) Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharm 186:612–619
O’Dell LE, Natividad LA, Pipkin JA, Roman F, Torres I, Juardo J, Torres OV, Friedman TC, Tenayuca JM, Nazarian A (2014) Enhanced nicotine self-administration and suppressed dopaminergic systems in a rat model of diabetes. Addict Biol 19:1006–1019
O’Dell LE, Khroyan TV (2009). Rodent models of nicotine reward: What do they tell us about tobacco abuse in humans? Pharmacology, Biochemistry and Behavior, 91: 481–488
O’Dell LE, Nazarian A (2016) Enhanced vulnerability to tobacco use in persons with diabetes: a behavioral and neurobiological framework. Prog Neuro-Psychopharmacol Biol Psychiatry 65:288–296
O’Dell LE, Torres OV, Natividad LA, Tejeda HA (2007) Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicol Teratol 29:17–22
Richardson JR, Pipkin JA, O’Dell LE, Nazarian A (2014) Insulin resistant rats display enhanced rewarding effects of nicotine. Drug Alcohol Depend 140:205–207
Shram MJ, Siu EC, Li Z, Tyndale RF, Le AD (2008) Interactions between age and the aversive effects of nicotine withdrawal under mecamylamine-percipitated and spontaneous conditions in male Wistart rats. Psychopharm (Berl) 198:181–190
Solberg LI, Desai JR, O’Connor PJ, Bishop DB, Devlin HM (2004) Diabetic patients who smoke: are they different? Ann Fam Med 2:26–32
Spangler JG, Summerson JH, Bell RA, Konen JC (2001) Smoking status and psychosocial variables in type 1 diabetes mellitus. Addict Behav 26:21–29
Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O’Dell LE (2009) Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharm (Berl) 206:303–312
Torres OV, Tejeda HA, Natividad LA, O’Dell LE (2008) Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav 90:658–653
Watkins SS, Stinus L, Koob GF, Markou A (2000) Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. J Phamacol Exp Ther 292:1053–1064
Zhang L, Dong Y, Doyon WM, Dani JA (2012) Withdrawal from chronic nicotine exposure alters dopamine signaling dynamics in the nucleus accumbens. Biol Psychiatry 71:184–191
Acknowledgements
The authors thank Tiahna Ontiveros, Robert Martinez, Emily Withrow, Christian Tejeda, and Victoria Edwards for their technical assistance. This research was supported by the American Diabetes Association (7-12-BS-135), National Institute on Drug Abuse (R15-DA040130; R01-DA021274; HHSN271201600057C), Diversity-promoting Institutions Drug Abuse Research Program (R24-DA029989), and SMART: MINDS Research Experience for Undergraduates Program (R25-DA033613).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pipkin, J.A., Cruz, B., Flores, R.J. et al. Both nicotine reward and withdrawal are enhanced in a rodent model of diabetes. Psychopharmacology 234, 1615–1622 (2017). https://doi.org/10.1007/s00213-017-4592-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4592-y