Abstract
Rationale
Mouse models of ethanol (EtOH) self-administration are useful to identify genetic and biological underpinnings of alcohol use disorder.
Objectives
These experiments developed a novel method of oral operant EtOH self-administration in mice without explicitly paired cues, food/water restriction, or EtOH fading.
Methods
Following magazine and lever training for 0.2 % saccharin (SAC), mice underwent nine weekly overnight sessions with lever pressing maintained by dipper presentation of 0, 3, 10, or 15 % EtOH in SAC or water vehicle. Ad libitum water was available from a bottle.
Results
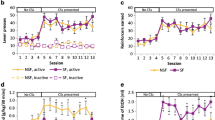
Water vehicle mice ingested most fluid from the water bottle in contrast to SAC vehicle mice, which despite lever pressing demands, drank most of their fluid from the liquid dipper. Although EtOH in SAC vehicle mice showed concentration-dependent increases of g/kg EtOH intake, lever pressing decreased with increasing EtOH concentration and did not exceed that of SAC vehicle alone at any EtOH concentration. Mice reinforced with EtOH in water ingested less EtOH than mice reinforced with EtOH in SAC. EtOH in water mice, however, showed concentration-dependent increases in g/kg EtOH intake and lever presses. Fifteen percent EtOH in water mice showed significantly greater levels of lever pressing than water vehicle mice and a significant escalation of responding across weeks of exposure. Naltrexone pretreatment reduced EtOH self-administration and intake in these mice without altering responding in the vehicle control condition during the first hour of the session.
Conclusions
SAC facilitated EtOH intake but prevented observation of EtOH reinforcement. Water vehicle unmasked EtOH’s reinforcing effects.





Similar content being viewed by others
References
Augier E, Flanigan M, Dulman RS, et al. (2014) Wistar rats acquire and maintain self-administration of 20 % ethanol without water deprivation, saccharin/sucrose fading, or extended access training. Psychopharmacology 1–8. doi:10.1007/s00213-014-3605-3
Baum WM (1993) Performances on ratio and interval schedules of reinforcement: data and theory. J Exp Anal Behav 59:254–264
Bell RL, Rodd ZA, Lumeng L et al (2006) The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol 11:270–288
Bell RL, Rodd ZA, Schultz JA et al (2008) Effects of short deprivation and re-exposure intervals on the ethanol drinking behavior of selectively bred high alcohol-consuming rats. Alcohol 42:407–416. doi:10.1016/j.alcohol.2008.03.130
Blednov YA, Benavidez JM, Black M et al (2014) GABAA receptors containing ρ1 subunits contribute to in vivo effects of ethanol in mice. PLoS One 9, e85525. doi:10.1371/journal.pone.0085525
Browne JD, Soko AD, Fletcher PJ (2014) Responding for conditioned reinforcement in C57BL/6J and CD-1 mice, and Sprague-Dawley rats: effects of methylphenidate and amphetamine. Psychopharmacology (Berl)
Brunzell DH, Chang JR, Schneider D et al (2006) beta2-subunit-containing nicotinic acetylcholine receptors are involved in nicotine-induced increases in conditioned reinforcement but not progressive ratio responding for food in C57BL/6J mice. Psychopharmacology (Berl) 184:328–338
Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF (2001) Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav 70:515–530. doi:10.1016/S0091-3057(01)00676-1
Cannady R, Fisher KR, Durant B et al (2013) Enhanced AMPA receptor activity increases operant alcohol self-administration and cue-induced reinstatement. Addict Biol 18:54–65. doi:10.1111/adb.12000
Cason AM, Aston-Jones G (2013) Attenuation of saccharin-seeking in rats by orexin/hypocretin receptor 1 antagonist. Psychopharmacology (Berl) 228:499–507. doi:10.1007/s00213-013-3051-7
Cunningham CL, Fidler TL, Hill KG (2000) Animal models of alcohol’s motivational effects. Alcohol Res Health 24:85–92
Dager AD, Anderson BM, Stevens MC et al (2013) Influence of alcohol use and family history of alcoholism on neural response to alcohol cues in college drinkers. Alcohol Clin Exp Res 37:161–171. doi:10.1111/j.1530-0277.2012.01879.x
Dager AD, Anderson BM, Rosen R et al (2014) Functional magnetic resonance imaging (fMRI) response to alcohol pictures predicts subsequent transition to heavy drinking in college students. Addiction 109:585–595. doi:10.1111/add.12437
Davison C, Corwin G, McGowan T (1976) Alcohol-induced taste aversion in golden hamsters. J Stud Alcohol 37:606–610
Doyon WM, Dong Y, Ostroumov A et al (2013) Nicotine decreases ethanol-induced dopamine signaling and increases self-administration via stress hormones. Neuron 79:530–540. doi:10.1016/j.neuron.2013.06.006
Dudek BC (1982) Ethanol-induced conditioned taste aversions in mice that differ in neural sensitivity to ethanol. J Stud Alcohol 43:126–136
Duncan SC, Strycker LA, Duncan TE (2012) Alcohol use of African Americans and whites from ages 9–20: descriptive results form a longitudinal study. J Ethn Subst Abus 11:214–225. doi:10.1080/15332640.2012.701550
Elmer GI, Meisch RA, George FR (1986) Oral ethanol reinforced behavior in inbred mice. Pharmacol Biochem Behav 24:1417–1421
Ferster CB, Skinner BF (1957) Schedules of reinforcement. Appleton, New York
Ford MM (2014) Applications of schedule-induced polydipsia in rodents for the study of an excessive ethanol intake phenotype. Alcohol 48:265–276. doi:10.1016/j.alcohol.2014.01.005
Garland EL, Franken IH, Howard MO (2012) Cue-elicited heart rate variability and attentional bias predict alcohol relapse following treatment. Psychopharmacology (Berl) 222:17–26. doi:10.1007/s00213-011-2618-4
Gillespie NA, Lubke GH, Gardner CO et al (2012) Two-part random effects growth modeling to identify risks associated with alcohol and cannabis initiation, initial average use and changes in drug consumption in a sample of adult, male twins. Drug Alcohol Depend 123:220–228. doi:10.1016/j.drugalcdep.2011.11.015
Gonzales RA, Job MO, Doyon WM (2004) The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther 103:121–146. doi:10.1016/j.pharmthera.2004.06.002
Grant KA, Samson HH (1985) Oral self-administration of ethanol in free feeding rats. Alcohol 2:317–321
Grant KA, Leng X, Green HL et al (2008) Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res 32:1824–1838. doi:10.1111/j.1530-0277.2008.00765.x
Grimm JW, Hope BT, Wise RA, Shaham Y (2001) Incubation of cocaine craving after withdrawal. Nature 412:141–142. doi:10.1038/35084134
Hay RA, Jennings JH, Zitzman DL et al (2013) Specific and nonspecific effects of naltrexone on goal-directed and habitual models of alcohol seeking and drinking. Alcohol Clin Exp Res 37:1100–1110
Holdstock L, King AC, de Wit H (2000) Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res 24:789–794
Kamdar NK, Miller SA, Syed YM et al (2007) Acute effects of Naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology 192:207–217. doi:10.1007/s00213-007-0711-5
Kelley BM, Middaugh LD (1996) Ethanol self-administration and motor deficits in adults C57BL/6J mice exposed prenatally to cocaine. Pharmacol Biochem Behav 55:575–584
Khisti RT, Wolstenholme J, Shelton KL, Miles MF (2006) Characterization of the ethanol-deprivation effect in substrains of C57BL/6 mice. Alcohol 40:119–126
Kidorf M, Lang AR, Pelham WE (1990) Beverage preference, beverage type and subject gender as determinants of alcohol consumption in the laboratory. J Stud Alcohol 51:331–335
King AC, Byars JA (2004) Alcohol-induced performance impairment in heavy episodic and light social drinkers. J Stud Alcohol 65:27–36
King AC, Houle T, de Wit H et al (2002) Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res 26:827–835
King AC, de Wit H, McNamara PJ, Cao D (2011) Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry 68:389–399. doi:10.1001/archgenpsychiatry.2011.26
King AC, McNamara PJ, Hasin DS, Cao D (2014) Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biol Psychiatry 75:798–806. doi:10.1016/j.biopsych.2013.08.001
King AC, Hasin D, O’Connor SJ, McNamara PJ, Cao D (2015) A prospective 5-year re-examination of alcohol response in heavy drinkers progressing in alcohol use disorder. Biol Psychiatry. doi:10.1016/j.biopsych.2015.05.007
McBride WJ, Li TK (1998) Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol 12:339–369
McKee SA, Harrison EL, O’Malley SS et al (2009) Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry 66:185–190
Messier C, White NM (1984) Contingent and non-contingent actions of sucrose and saccharin reinforcers: effects on taste preference and memory. Physiol Behav 32:195–203
Middaugh LD, Kelley BM (1999) Operant ethanol reward in C57BL/6 mice: influence of gender and procedural variables. Alcohol 17:185–194
Middaugh LD, Kelley BM, Bandy AL, McGroarty KK (1999a) Ethanol consumption by C57BL/6 mice: influence of gender and procedural variables. Alcohol 17:175–183
Middaugh LD, Kelley BM, Cuison ER, Groseclose CH (1999b) Naltrexone effects on ethanol reward and discrimination in C57BL/6 mice. Alcohol Clin Exp Res 23:456–464
Middaugh LD, Lee AM, Bandy AL (2000) Ethanol reinforcement in nondeprived mice: effect of abstinence and naltrexone. Alcohol Clin Exp Res 24:1172–1179
Navarrete F, Rubio G, Manzanares J (2013) Effects of naltrexone plus topiramate on ethanol self-administration and tyrosine hydroxylase gene expression changes. Addict Biol. doi:10.1111/adb.12058
O’Connor RM, Colder CR (2009) Influence of alcohol use experience and motivational drive on college students’ alcohol-related cognition. Alcohol Clin Exp Res 33:1430–1439. doi:10.1111/j.1530-0277.2009.00973.x
O’Malley SS, Rounsaville BJ, Farren C et al (2003) Initial and maintenance naltrexone treatment for alcohol dependence using primary care vs specialty care: a nested sequence of 3 randomized trials. Arch Intern Med 163:1695–1704
O’Malley SS, Garbutt JC, Gastfriend DR et al (2007) Efficacy of extended-release naltrexone in alcohol-dependent patients who are abstinent before treatment. J Clin Psychopharmacol 27:507–512
Olsen CM, Winder DG (2009) Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacology 34:1685–1694. doi:10.1038/npp.2008.226
Olsen CM, Winder DG (2012) Stimulus dynamics increase the self-administration of compound visual and auditory stimuli. Neurosci Lett 511:8–11. doi:10.1016/j.neulet.2011.12.068
Petit G, Kornreich C, Verbanck P, Campanella S (2013) Gender differences in reactivity to alcohol cues in binge drinkers: a preliminary assessment of event-related potentials. Psychiatry Res 209:494–503. doi:10.1016/j.psychres.2013.04.005
Phillips TJ, Wenger CD, Dorow JD (1997) Naltrexone effects on ethanol drinking acquisition and on established ethanol consumption in C57BL/6J mice. Alcohol Clin Exp Res 21:691–702
Prescott CA, Kendler KS (1999) Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry 156:34–40
Regier PS, Carroll ME, Meisel RL (2012) Cocaine-induced c-Fos expression in rats selectively bred for high or low saccharin intake and in rats selected for high or low impulsivity. Behav Brain Res 233:271–279. doi:10.1016/j.bbr.2012.05.021
Rhodes JS, Crabbe JC (2003) Progress towards finding genes for alcoholism in mice. Clin Neurosci Res 3:315–323. doi:10.1016/j.cnr.2003.10.012
Rhodes JS, Best K, Belknap JK et al (2005) Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav 84:53–63. doi:10.1016/j.physbeh.2004.10.007
Risinger FO, Brown MM, Doan AM, Oakes RA (1998) Mouse strain differences in oral operant ethanol reinforcement under continuous access conditions. Alcohol Clin Exp Res 22:677–684
Rodd ZA, Bell RL, Kuc KA et al (2002) Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: II. Adult exposure. Alcohol Clin Exp Res 26:1642–1652
Rodd ZA, Bell RL, Kuc KA et al (2003) Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology 28:1614–1621
Ryabinin AE, Galvan-Rosas A, Bachtell RK, Risinger FO (2003) High alcohol/sucrose consumption during dark circadian phase in C57BL/6J mice: involvement of hippocampus, lateral septum and urocortin-positive cells of the Edinger-Westphal nucleus. Psychopharmacology 165:296–305. doi:10.1007/s00213-002-1284-y
Samson HH (1986) Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res 10:436–442
Samson HH, Pfeffer AO, Tolliver GA (1988) Oral ethanol self-administration in rats: models of alcohol-seeking behavior. Alcohol Clin Exp Res 12:591–598
Santos N, Chatterjee S, Henry A et al (2013) The α5 neuronal nicotinic acetylcholine receptor subunits plays an important role in the sedative effects of ethanol but does not modulate consumption in mice. Alcohol Clin Exp Res 37:655–662. doi:10.1111/acer.12009
Schuckit MA, Smith TL (1996) An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry 53:202–210
Sharko AC, Hodge CW (2008) Differential modulation of ethanol-induced sedation and hypnosis by metabotropic glutamate receptor antagonists in C57BL/6J mice. Alcohol Clin Exp Res 32:67–76
Sjoerds Z, van den Brink W, Beekman AT et al (2014) Cue reactivity is associated with duration and severity of alcohol dependence: an fMRI study. PLoS One 9, e84560. doi:10.1371/journal.pone.0084560
Spanagel R, Hölter SM (1999) Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol 34:231–243
Tabakoff B, Hoffman PL (2000) Animal models in alcohol research. Alcohol Res Health 24:77–84
Tabakoff B, Hoffman PL (2013) The neurobiology of alcohol consumption and alcoholism: an integrative history. Pharmacol Biochem Behav 113:20–37. doi:10.1016/j.pbb.2013.10.009
van Erp AMM, Miczek KA (2007) Increased accumbal dopamine during daily alcohol consumption and subsequent aggressive behavior in rats. Psychopharmacology 191:679–688. doi:10.1007/s00213-006-0637-3
Wang D, Raehal KM, Lin ET, Lowery JJ, Kieffer BL, Bilsky EJ, Sadee W (2004) Basal signaling activity of μ opioid receptor in mouse brain: role of narcotic dependence. J Pharmacol Exp Ther 308:512–520. doi:10.1124/jpet.103.054049
World Health Organization (2011) Global status report on alcohol and health
Acknowledgments
We wish to thank Meredith A. Beck, Jennifer M. Lee, and Galina Slavova-Hernandez for technical assistance. Alexandra M. Stafford and Shawn M. Anderson were supported by NIH training grant T32DA007027 to William L. Dewey. Darlene H. Brunzell was supported in part by NIH P50 AA022537 to Kenneth S. Kendler and NIH R01 DA031289 to Dr. Brunzell. Dr. Shelton was supported in part by NIH R01 DA020553. This work was supported by a Virginia Commonwealth University Alcohol Research Center pilot award to Darlene H. Brunzell, NIH P20AA017828 to M.F. Miles.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
(PDF 55 kb)
Online Resource 2
(PDF 117 kb)
Online Resource 3
(PDF 587 kb)
Online Resource 4
(PDF 837 kb)
Rights and permissions
About this article
Cite this article
Stafford, A.M., Anderson, S.M., Shelton, K.L. et al. Oral operant ethanol self-administration in the absence of explicit cues, food restriction, water restriction and ethanol fading in C57BL/6J mice. Psychopharmacology 232, 3783–3795 (2015). https://doi.org/10.1007/s00213-015-4040-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4040-9