Abstract
Rationale
Evidence suggests that neuronal nicotinic acetylcholine receptor (nAChR) ligand lobeline has antidepressant-like properties.
Objectives
The present study investigated the effects of lobeline on nicotine withdrawal-induced depression-like behavior.
Methods
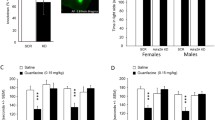
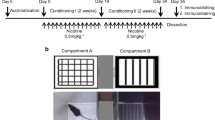
Adult C57BL/6J mice were exposed to nicotine (200 μg/ml) in drinking solution for 3 weeks. During withdrawal, depression-like behavior was measured by the forced swim test (FST). We also determined norepinephrine (NE) levels in the prefrontal cortex (PFC) and hippocampus during nicotine withdrawal. Furthermore, we determined the effects of repeated treatment with lobeline or a selective α4β2 nAChR ligand 3-(pyridine-3́-yl)-cytisine on brain-derived neurotrophic factor (BDNF) and phosphorylated cAMP-responsive element binding (p-CREB) protein expression in the hippocampus.
Results
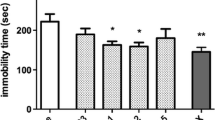
Withdrawal from chronic nicotine increased immobility time in the FST, a measure for depression-like behavior. Pretreatment with lobeline significantly decreased immobility time during nicotine withdrawal. In addition, pretreatment with lobeline attenuated nicotine withdrawal-induced increased NE levels in the PFC and hippocampus. Further, repeated treatment with lobeline or 3-(pyridine-3́-yl)-cytisine decreased immobility time in the FST and reduced withdrawal-induced increased BDNF and p-CREB expression in the hippocampus.
Conclusions
Taken together, our results indicate that lobeline attenuated nicotine withdrawal-induced depression-like behavior likely by targeting brain nAChRs, noradrenergic neurotransmission, and/or hippocampal BDNF. Thus, lobeline may have some potential to prevent smoking relapse by counteracting nicotine withdrawal-induced depression in humans.








Similar content being viewed by others
References
Andreasen JT, Nielsen EØ, Redrobe JP (2009) Chronic oral nicotine increases brain [3H] epibatidine binding and responsiveness to antidepressant drugs, but not nicotine, in the mouse forced swim test. Psychopharmacology (Berl) 205:517–528
Bell RL, Eilier BA, Cook JB, Rahman S (2009) Nicotinic receptor ligands reduce ethanol intake by high alcohol-drinking HAD-2 rats. Alcohol 43:581–592
Benowitz NL (2010) Nicotine addiction. N Engl J Med 362:2295–2303
Bhang SY, Choi SW, Ahn JH (2010) Changes in plasma brain-derived neurotrophic factor levels in smokers after smoking cessation. Neurosci Lett 468:7–11
Brunzell DH, Russell DS, Picciotto MR (2003) In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. J Neurochem 84:1431–1441
Campbell S, MacQueen G (2004) The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci 29:417–426
Chen MJ, Nguyen TV, Pike CJ, Russo-Neustadt AA (2007) Norepinephrine induces BDNF and activates the PI-3K and MAPK cascades in embryonic hippocampal neurons. Cell Signal 19:114–128
Damaj M, Patrick G, Creasy K, Martin B (1997) Pharmacology of lobeline, a nicotinic receptor ligand. J Pharmacol Exp Ther 282:410–419
Duman RS, Aghajanian GK (2012) Synaptic dysfunction in depression: potential therapeutic targets. Science 338:68–72
Dwoskin LP, Crooks PA (2002) A novel mechanism of action and potential use for lobeline as a treatment for psychostimulant abuse. Biochem Pharmacol 63:89–98
Filip M, Faron-Górecka A, Kuśmider M, Gołda A, Frankowska M, Dziedzicka-Wasylewska M (2006) Alterations in BDNF and trkB mRNAs following acute or sensitizing cocaine treatments and withdrawal. Brain Res 1071:218–225
Franklin KBG, Paxinos G (2007) The mouse brain in stereotaxic coordinates, 3rd edn. Academic Press, Inc., New York
Gäddnäs H, Pietilä K, Ahtee L (2000) Effects of chronic oral nicotine treatment and its withdrawal on locomotor activity and brain monoamines in mice. Behav Brain Res 113:65–72
Gallardo KA, Leslie FM (1998) Nicotine-stimulated release of [3H]norepinephrine from fetal rat locus coeruleus cells in culture. J Neurochem 70:663–670
George MS, Ketter TA, Post RM (1994) Prefrontal cortex dysfunction in clinical depression. Depression 2:59–72
Glover ED, Leischow SJ, Rennard SI, Glover PN, Daughton D, Quiring JN, Schneider FH, Mione PJ (1998) A smoking cessation trial with lobeline sulfate: a pilot study. Am J Health Behav 22:62–74
Grabus S, Martin B, Imad Damaj M (2005) Nicotine physical dependence in the mouse: involvement of the α7 nicotinic receptor subtype. Eur J Pharmacol 515:90–93
Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y (2003) Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci 23:742–747
Harrod SB, Van Horn ML (2009) Sex differences in tolerance to the locomotor depressant effects of lobeline in periadolescent rats. Pharmacol Biochem Behav 94:296–304
Harrod SB, Dwoskin LP, Crooks PA, Klebaur JE, Bardo MT (2001) Lobeline attenuates d-methamphetamine self-administration in rats. J Pharmacol Exp Ther 298:172–179
Hughes, J.R., Stead, L.F., Lancaster, T. (2007) Antidepressants for smoking cessation. Cochrane Database Syst Rev 1:CD000031.
Jackson KJ, Martin BR, Changeux JP, Damaj MI (2008) Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther 325:302–312
Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI (2009) The role of α6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J Pharmacol Exp Ther 331:547–554
Kim TS, Kim DJ, Lee H, Kim YK (2007) Increased plasma brain-derived neurotrophic factor levels in chronic smokers following unaided smoking cessation. Neurosci Lett 423:53–57
Kivinummi T, Kaste K, Rantamäki T, Castrén E, Ahtee L (2011) Alterations in BDNF and phospho-CREB levels following chronic oral nicotine treatment and its withdrawal in dopaminergic brain areas of mice. Neurosci Lett 491:108–112
Klein LC, Stine MM, Vandenbergh DJ, Whetzel CA, Kamens HM (2004) Sex differences in voluntary oral nicotine consumption by adolescent mice: a dose–response experiment. Pharmacol Biochem Behav 78:13–25
Krishnan V, Nestler EJ (2008) The molecular neurobiology of depression. Nature 455:894–902
Mannucci C, Tedesco M, Bellomo M, Caputi A, Calapai G (2006) Long-term effects of nicotine on the forced swimming test in mice: an experimental model for the study of depression caused by smoke. Neurochem Int 49:481–486
McClung CA, Nestler EJ (2007) Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology 33:3–17
Miller DK, Crooks P, Dwoskin LP (2000) Lobeline inhibits nicotine-evoked [3H]dopamine overflow from rat striatal slices and nicotine-evoked 86Rb+ efflux from thalamic synaptosomes. Neuropharmacology 39:2654–2662
Miller DK, Crooks PA, Teng L, Witkin JM, Munzar P, Goldberg SR, Acri JB, Dwoskin LP (2001) Lobeline inhibits the neurochemical and behavioral effects of amphetamine. J Pharmacol Exp Ther 296:1023–1034
Miller DK, Harrod SB, Green TA, Wong MY, Bardo MT, Dwoskin LP (2003) Lobeline attenuates locomotor stimulation induced by repeated nicotine administration in rats. Pharmacol Biochem Behav 74:279–286
Mineur YS, Eibl C, Young G, Kochevar C, Papke RL, Gündisch D, Picciotto MR (2009) Cytisine-based nicotinic partial agonists as novel antidepressant compounds. J Pharmacol Exp Ther 329:377–386
Nguyen HN, Rasmussen BA, Perry DC (2003) Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J Pharmacol Exp Ther 307:1090–1097
Parker MJ, Beck A, Luetje CW (1998) Neuronal nicotinic receptor β2 and β4 subunits confer large differences in agonist binding affinity. Mol Pharmacol 54:1132–1139
Pietilä K, Laakso I, Ahtee L (1995) Chronic oral nicotine administration affects the circadian rhythm of dopamine and 5-hydroxytryptamine metabolism in the striata of mice. Naunyn-Schmiedeberg Arch Pharmacol 353:110–115
Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732
Rabenstein RL, Caldarone BJ, Picciotto MR (2006) The nicotinic antagonist mecamylamine has antidepressant-like effects in wild-type but not beta2- or alpha7-nicotinic acetylcholine receptor subunit knockout mice. Psychopharmacology (Berl) 189:395–401
Rahman S, Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT (2008) The novel nicotinic receptor antagonist N,N′-dodecane-1,12-diyl-bis-3-picolinium dibromide decreases nicotine-induced dopamine metabolism in rat nucleus accumbens. Eur J Pharmacol 601:103–105
Ressler KJ, Nemeroff CB (2000) Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety 12:2–19
Ribeiro-Carvalho A, Lima CS, Medeiros AH, Siqueira NR, Filgueiras CC, Manhaes AC, Abreu-Villaça Y (2009) Combined exposure to nicotine and ethanol in adolescent mice: effects on the central cholinergic systems during short and long term withdrawal. Neurosci 162:1174–1186
Robinson SF, Marks MJ, Collins AC (1996) Inbred mouse strains vary in oral self-selection of nicotine. Psychopharmacology (Berl) 124:332–339
Roni MA, Rahman S (2011) Neuronal nicotinic receptor antagonist reduces anxiety-like behavior in mice. Neurosci Lett 504:237–241
Roni MA, Rahman S (2013) Antidepressant-like effects of lobeline in mice: behavioral, neurochemical, and neuroendocrine evidence. Prog Neuropsychopharmacol Biol Psychiatry 41:44–51
Sajja RK, Rahman S (2011) Lobeline and cytisine reduce voluntary ethanol drinking behavior in male C57BL/6J mice. Prog Neuro-Psychopharmacol Biol Psychiatry 35:257–264
Sajja RK, Rahman S (2012) Neuronal nicotinic receptor ligands modulate chronic nicotine-induced ethanol consumption in C57BL/6J mice. Pharmacol Biochem Behav 102:36–43
Sajja RK, Dwivedi C, Rahman S (2010) Nicotinic ligands modulate ethanol-induced dopamine function in mice. Pharmacology 86:168–173
Salas R, Pieri F, De Biasi M (2004) Decreased signs of nicotine withdrawal in mice null for the β4 nicotinic acetylcholine receptor subunit. J Neurosci 24:10035–10039
Sanberg PR, Vindrola-Padros C, Shytle RD (2012) Translating laboratory discovery to the clinic: from nicotine and mecamylamine to tourettes, depression, and beyond. Physiology Behav 107:801–808
Semba J, Wakuta M, Maeda J, Suhara T (2004) Nicotine withdrawal induces subsensitivity of hypothalamic–pituitary–adrenal axis to stress in rats: implications for precipitation of depression during smoking cessation. Psychoneuroendocrinology 29:215–226
Sparks JA, Pauly JR (1999) Effects of continuous oral nicotine administration on brain nicotinic receptors and responsiveness to nicotine in C57Bl/6 mice. Psychopharmacology (Berl) 14:145–153
Stolerman IP, Garcha HS, Mirza NR (1995) Dissociations between the locomotor stimulant and depressant effects of nicotinic agonists in rats. Psychopharmacology (Berl) 117:430–437
Tani Y, Saito K, Tsuneyoshi A, Imoto M, Ohno T (1997) Nicotinic acetylcholine receptor (nACh-R) agonist-induced changes in brain monoamine turnover in mice. Psychopharmacology (Berl) 129:225–232
Watkins SS, Stinus L, Koob GF, Markou A (2000) Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J Pharmacol Exp Ther 292:1053–1064
Wilkinson JL, Palmatier MI, Bevins RA (2006) Preexposure to nicotine alters the subsequent locomotor stimulant effects of bupropion in rats. Nicotine Tob Res 8:141–146
Xu Y, Ku B, Tie L, Yao H, Jiang W, Ma X, Li X (2006) Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res 1122:56–64
Zaniewska M, McCreary AC, Wydra K, Filip M (2010) Effects of serotonin 5-HT 2 receptor ligands on depression-like behavior during nicotine withdrawal. Neuropharmacology 58:1140–1146
Acknowledgments
The authors wish to thank Dr. Chandradhar Dwivedi and Dr. Ruth Guillermo for their help in Western blot studies. The anti-β-tubulin antibody developed by Dr. Michael Klymkowsky was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biology, Iowa City, IA 52242. This study was supported in part by the funds from the College of Pharmacy and grants from SDSU Research Foundation and RSSF (SR). All experiments were in compliance with the National Institutes of Health guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at South Dakota State University in the USA.
Conflict of interest
The authors declare that there is no conflict of interest related to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roni, M.A., Rahman, S. The effects of lobeline on nicotine withdrawal-induced depression-like behavior in mice. Psychopharmacology 231, 2989–2998 (2014). https://doi.org/10.1007/s00213-014-3472-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3472-y