Abstract
Rationale
Ziprasidone is an atypical antipsychotic recommended to be administered twice daily.
Objectives
The purpose of this study was to investigate whether occupancy of the dopamine D2/3 receptors by ziprasidone is maintained across a day employing a within subject design.
Methods
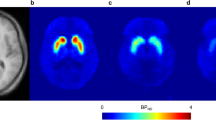
Positron emission tomography (PET) scans with [11C]-raclopride were performed in 12 patients with schizophrenia while treated with ziprasidone 60 mg twice daily. Each patient completed [11C]-raclopride PET scans at 5, 13 and 23 h after the last dose of ziprasidone. Dopamine D2/3 receptor occupancy was estimated with reference to binding potential data of 44 age- and sex-matched control subjects in the caudate, putamen and ventral striatum.
Results
Eleven scans were available at each time point, and the mean occupancies at 5-, 13- and 23-h scans were 66, 39 and 2 % in the putamen; 62, 35 and −6 % in the caudate; and 68, 47 and 11 % in the ventral striatum, respectively. The time-course of receptor occupancy across the regions indicated an occupancy half-life of 8.3 h. The serum level of ziprasidone associated with 50 % D2/3 receptors occupancy was estimated to be 204 nmol/L (84 ng/ml). Prolactin levels were highest at 5-h post-dose and none showed hyperprolactinemia at 23-h scans.
Conclusions
The absence of ziprasidone striatal D2/3 receptor binding 23 h after taking 60 mg under steady-state conditions is consistent with its peripheral half-life. The results support our earlier report that ziprasidone 60 mg administered twice daily appears to be the minimal dose expected to achieve therapeutic central dopamine D2/3 receptor occupancy (i.e. 60 %).
Clinical Trials Registration: 24-Hour Time Course of Striatal Dopamine D2 Receptor Occupancy of Ziprasidone: A PET Study, www.clinicaltrials.gov/ct2/show/NCT00818298, NCT00818298



Similar content being viewed by others
References
Bench C, Lammerstma A, Grasby P, Dolan R, Frackowiak R, Warrington S, Boyce M, Gunn K, Brannick L (1996) The time course of binding to striatal dopamine D < sub > 2</sub > receptors by the neuroleptic ziprasidone (CP-88,059–01) determined by positron emission tomography. Psychopharmacology 124:141–147
Bench C, Lammertsma A, Dolan R, Grasby P, Warrington S, Gunn K, Cuddigan M, Turton D, Osman S, Frackowiak R (1993) Dose dependent occupancy of central dopamine D2 receptors by the novel neuroleptic CP-88,059–01: a study using positron emission tomography and 11C-raclopride. Psychopharmacology 112:308–314
Caley CF, Cooper CK (2002) Ziprasidone: the fifth atypical antipsychotic. Ann Pharmacother 36:839–851
Cherma MD, Reis M, Hagg S, Ahlner J, Bengtsson F (2008) Therapeutic drug monitoring of ziprasidone in a clinical treatment setting. Ther Drug Monit 30:682–688
Citrome L (2009) Using oral ziprasidone effectively: the food effect and dose–response. Adv Ther 26:739–748
Citrome L, Reist C, Palmer L, Montejano LB, Lenhart G, Cuffel B, Harnett J, Sanders KN (2009) Impact of real-world ziprasidone dosing on treatment discontinuation rates in patients with schizophrenia or bipolar disorder. Schizophr Res 115:115–120
Corripio I, Catafau AM, Perez V, Puigdemont D, Mena E, Aguilar Y, Carrió I, Álvarez E (2005) Striatal dopaminergic D2 receptor occupancy and clinical efficacy in psychosis exacerbation: a 123I-IBZM study with ziprasidone and haloperidol. Prog Neuro-Psychopharmacol Biol Psychiatry 29:91–96
Daniel DG, Zimbroff DL, Potkin SG, Reeves KR, Harrigan EP, Lakshminarayanan M (1999) Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Ziprasidone Study Group. Neuropsychopharmacology 20:491–505
Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G (1992) Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry 49:538–544
Farde L, Wiesel FA, Halldin C, Sedvall G (1988) Central D2-dopamine receptor occupancy in schizophrenic patients treated with antipsychotic drugs. Arch Gen Psychiatry 45:71–76
Fischman AJ, Bonab AA, Babich JW, Alpert NM, Rauch SL, Elmaleh DR, Shoup TM, Williams SA, Rubin RH (1996) Positron emission tomographic analysis of central 5-hydroxytryptamine2 receptor occupancy in healthy volunteers treated with the novel antipsychotic agent, ziprasidone. J Pharmacol Exp Ther 279:939–947
Frankle WG, Lombardo I, Kegeles LS, Slifstein M, Martin JH, Huang Y, Hwang DR, Reich E, Cangiano C, Gil R, Abi-Dargham A, Laruelle M (2011) Measurement of the serotonin 1A receptor availability in patients with schizophrenia during treatment with the antipsychotic medication ziprasidone. J Psychopharmacol 25:734–743
Graff-Guerrero A, Willeit M, Ginovart N, Mamo D, Mizrahi R, Rusjan P, Vitcu I, Seeman P, Wilson AA, Kapur S (2008) Brain region binding of the D(2/3) agonist [(11)C]-(+)-PHNO and the D(2/3) antagonist [(11)C]raclopride in healthy humans. Hum Brain Mapp 29:400–410
Grunder G, Fellows C, Janouschek H, Veselinovic T, Boy C, Brocheler A, Kirschbaum KM, Hellmann S, Spreckelmeyer KM, Hiemke C, Rosch F, Schaefer WM, Vernaleken I (2008) Brain and plasma pharmacokinetics of aripiprazole in patients with schizophrenia: an [18F]fallypride PET study. Am J Psychiatry 165:988–995
Gründer G, Hiemke C, Paulzen M, Veselinovic T, Vernaleken I (2011) Therapeutic plasma concentrations of antidepressants and antipsychotics: lessons from PET imaging. Pharmacopsychiatry 44:236–248
Guy W (1976) Clinical global impressions. ECDEU Assessment Manual for Psychopharmacology rDPNA-NIoMHR, MD, In, pp 218–222
Heard K, Krier S, Zahniser NR (2008) Administration of ziprasidone for 10 days increases cocaine toxicity in mice. Hum Exp Toxicol 27:499–503
Hiemke C, Baumann P, Bergemann N, Conca A, Dietmaier O, Egberts K, Fric M, Gerlach M, Greiner C, Gründer G, Haen E, Havemann-Reinecke U, Jaquenoud Sirot E, Kirchherr H, Laux G, Lutz UC, Messer T, Müller MJ, Pfuhlmann B, Rambeck B, Riederer P, Schoppek B, Stingl J, Uhr M, Ulrich S, Waschgler R, Zernig G (2011) AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry 44:195–235
Hirsch SR, Kissling W, Bauml J, Power A, O'Connor R (2002) A 28-week comparison of ziprasidone and haloperidol in outpatients with stable schizophrenia. J Clin Psychiatry 63:516–523
Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S (2012) The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry 69:776–786
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE (2007) Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27:1533–1539
Kapur S, Remington G, Zipursky RB, Wilson AA, Houle S (1995) The D2 dopamine receptor occupancy of risperidone and its relationship to extrapyramidal symptoms: a PET study. Life Sci 57: PL103-7.
Kapur S, Zipursky R, Jones C, Remington G, Houle S (2000a) Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry 157:514–520
Kapur S, Zipursky R, Jones C, Shammi CS, Remington G, Seeman P (2000b) A positron emission tomography study of quetiapine in schizophrenia: a preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch Gen Psychiatry 57:553–559
Kapur S, Zipursky RB, Jones C, Remington GJ, Wilson AA, DaSilva J, Houle S (1996) The D2 receptor occupancy profile of loxapine determined using PET. Neuropsychopharmacology 15:562–566
Kapur S, Zipursky RB, Remington G (1999) Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry 156:286–293
Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ, Frackowiak RSJ (1996) Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab 16:42–52
Mamo D, Kapur S, Shammi CM, Papatheodorou G, Mann S, Therrien F, Remington G (2004) A PET study of dopamine D2 and serotonin 5-HT2 receptor occupancy in patients with schizophrenia treated with therapeutic doses of ziprasidone. Am J Psychiatry 161:818–825
Mamo DC, Uchida H, Vitcu I, Barsoum P, Gendron A, Goldstein J, Kapur S (2008) Quetiapine extended-release versus immediate-release formulation: a positron emission tomography study. J Clin Psychiatry 69:81–86
Nordstrom AL, Farde L, Nyberg S, Karlsson P, Halldin C, Sedvall G (1995) D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: a PET study of schizophrenic patients. Am J Psychiatry 152:1444–1449
Pickar D, Su TP, Weinberger DR, Coppola R, Malhotra AK, Knable MB, Lee KS, Gorey J, Bartko JJ, Breier A, Hsiao J (1996) Individual variation in D2 dopamine receptor occupancy in clozapine-treated patients. Am J Psychiatry 153:1571–1578
Pilowsky LS, Costa DC, Ell PJ, Murray RM, Verhoeff NP, Kerwin RW (1993) Antipsychotic medication, D2 dopamine receptor blockade and clinical response: a 123I IBZM SPET (single photon emission tomography) study. Psychol Med 23:791–797
Seeman P (2011) All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2(high) receptors. CNS Neurosci Ther 17:118–132
Simpson GM, Weiden P, Pigott T, Murray S, Siu CO, Romano SJ (2005) Six-month, blinded, multicenter continuation study of ziprasidone versus olanzapine in schizophrenia. Am J Psychiatry 162:1535–1538
Stahl SM, Malla A, Newcomer JW, Potkin SG, Weiden PJ, Harvey PD, Loebel A, Watsky E, Siu CO, Romano S (2010) A post hoc analysis of negative symptoms and psychosocial function in patients with schizophrenia: a 40-week randomized, double-blind study of ziprasidone versus haloperidol followed by a 3-year double-blind extension trial. J Clin Psychopharmacol 30:425–430
Stahl SM, Shayegan DK (2003) The psychopharmacology of ziprasidone: receptor-binding properties and real-world psychiatric practice. J Clin Psychiatry 64(Suppl 19):6–12
Suhara T, Okauchi T, Sudo Y, Takano A, Kawabe K, Maeda J, Kapur S (2002) Clozapine can induce high dopamine D(2) receptor occupancy in vivo. Psychopharmacology (Berl) 160:107–112
Tauscher J, Jones C, Remington G, Zipursky RB, Kapur S (2002) Significant dissociation of brain and plasma kinetics with antipsychotics. Mol Psychiatry 7:317–321
Uchida H, Graff-Guerrero A, Mulsant BH, Pollock BG, Mamo DC (2009) Long-term stability of measuring D(2) receptors in schizophrenia patients treated with antipsychotics. Schizophr Res 109:130–133
Uchida H, Takeuchi H, Graff-Guerrero A, Suzuki T, Watanabe K, Mamo DC (2011) Predicting dopamine D2 receptor occupancy from plasma levels of antipsychotic drugs: a systematic review and pooled analysis. J Clin Psychopharmacol: 318–25
Vernaleken I, Fellows C, Janouschek H, Brocheler A, Veselinovic T, Landvogt C, Boy C, Buchholz HG, Spreckelmeyer K, Bartenstein P, Cumming P, Hiemke C, Rosch F, Schafer W, Wong DF, Grunder G (2008) Striatal and extrastriatal D2/D3-receptor-binding properties of ziprasidone: a positron emission tomography study with [18F]Fallypride and [11C]raclopride (D2/D3-receptor occupancy of ziprasidone). J Clin Psychopharmacol 28:608–617
Vogel F, Gansmüller R, Leiblein T, Dietmaier O, Wassmuth H, Gründer G, Hiemke C (2009) The use of ziprasidone in clinical practice: analysis of pharmacokinetic and pharmacodynamic aspects from data of a drug monitoring survey. Eur Psychiatry 24:143–148
Wessels AM, Bies RR, Pollock BG, Schneider LS, Lieberman JA, Stroup S, Li CH, Coley K, Kirshner MM, Marder SR (2011) Population pharmacokinetic modeling of ziprasidone in patients with schizophrenia from the CATIE study. J Clin Pharmacol 51:1587–1591
Acknowledgments
The authors gratefully acknowledge Alan Wilson, PhD, for supervising the radiochemical syntheses and Sylvain Houle, MD, PhD; Armando Garcia, BSc; Wanna Mar MSc (all are from the Centre for Addiction and Mental Health, Toronto, ON, Canada) for their assistance. They report no financial or other relationships with other organization, including pharmaceutical companies.
Conflicts of interest
This study was supported by an investigator initiated research grant from Pfizer Canada Inc. Dr. Suzuki has received manuscript or speaker’s fees from Dainippon Sumitomo Pharma, Eli Lilly, Astellas, Meiji Seika Pharma and Novartis Pharma. Dr. Graff-Guerrerro currently receives research support from the following external funding agencies: Canadian Institutes of Health Research (CIHR), the US National Institute of Health (NIH), Ontario Mental Health Foundation, Mexico ICyTDF and CONACyT. Dr. Uchida has received grants from Pfizer and Dainippon-Sumitomo Pharma, and speaker’s honoraria from Otsuka Pharmaceutical, Eli Lilly, Novartis Pharma, Shionogi, GlaxoSmithKline, Yoshitomi Yakuhin, Dainippon-Sumitomo Pharma and Janssen Pharmaceutical within the past 2 years. Dr. Gary Remington, in the last 3 years, has received research support (Principal Investigator) from the following external funding agencies: CIHR, Research Hospital Fund—Canada Foundation for Innovation GUF—CFI), Schizophrenia Society of Ontario (SSO) and the Canadian Diabetes Association (CDA). He has also received support from Novartis Canada Medicure Inc., and Neurocrine Bioscience. He has received consultant fees from Laboratorios Farmacduticos ROVI, Novartis and Roche, as well as speaker's fees from Novartis. He holds no commercial investments in any pharmaceutical company. Mr. Caravaggio and Ms. Borlido have nothing to disclose. Dr. Pollock receives research support from the US NIH, CIHR, American Psychiatric Association and the Foundation of the Centre for Addiction and Mental Health. Dr Mulsant currently receives research support from the CIHR, the US NIH, Bristol-Myers Squibb (medications for a NIH-funded clinical trial) and Pfizer (medications for a NIH-funded clinical trial). He directly owns stocks of General Electric (less than $5, 000). Within the past five years he has also received some grant support from Eli Lilly (medications for a NIH-funded clinical trial) and Janssen and some travel support from Roche. Dr. DeLuca has nothing to disclose. Dr. Ismail received support as consultant for Sunovion, BMS, Pfizer and Lundbeck. He also received research support form Pfizer and has honoraria from Otsuka, Lundbeck and Pfizer. Dr. Mamo currently receives research support from the following external funding agencies: CIHR, the US NIH, Ontario Ministry of Health and Long Term Care. He has also received grants or consultant fees from the Bristol-Myers Squibb and Pfizer and has received speaker’s honoraria from AstraZeneca in the past 3 years.
Author information
Authors and Affiliations
Corresponding author
Additional information
Previous Presentation: Part of this work was presented at the Society of Biological Psychiatry 66th Annual Scientific Convention, in San Francisco May 12–14, 2011
Rights and permissions
About this article
Cite this article
Suzuki, T., Graff-Guerrero, A., Uchida, H. et al. Dopamine D2/3 occupancy of ziprasidone across a day: a within-subject PET study. Psychopharmacology 228, 43–51 (2013). https://doi.org/10.1007/s00213-013-3012-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3012-1