Abstract
Background and objectives
The idea that nucleus accumbens (Acb) dopamine transmission contributes to the neural mediation of reward, at least in a general sense, has achieved wide acceptance. Nevertheless, debate remains over the precise nature of dopamine’s role in reward and even over the nature of reward itself. In the present article, evidence is reviewed from studies of food intake, feeding microstructure, instrumental responding for food reinforcement, and dopamine efflux associated with feeding, which suggests that reward processing in the Acb is best understood as an interaction among distinct processes coded by discrete neurotransmitter systems.
Results
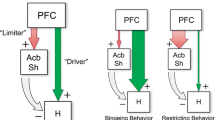
In agreement with several theories of Acb dopamine function, it is proposed here that allocation of motor effort in seeking food or food-associated conditioned stimuli can be dissociated from computations relevant to the hedonic evaluation of food during the consummatory act. The former appears to depend upon Acb dopamine transmission and the latter upon striatal opioid peptide release. Moreover, dopamine transmission may play a role in ‘stamping in’ associations between motor acts and goal attainment and perhaps also neural representations corresponding to rewarding outcomes. Finally, evidence is reviewed that amino acid transmission specifically in the Acb shell acts as a central ‘circuit breaker’ to flexibly enable or terminate the consummatory act, via descending connections to hypothalamic feeding control systems.
Conclusions
The heuristic framework outlined above may help explain why dopamine-compromising manipulations that strongly diminish instrumental goal-seeking behaviors leave consummatory activity relatively unaffected.



Similar content being viewed by others
Notes
For the purposes of this review, we will use the terms ‘appetitive’ and ‘consummatory’ to distinguish behaviors leading up to goal attainment from those that involve actual commerce with the goal object (Craig 1918). Unless otherwise specified, the term ‘appetitive phase’ is meant, in a broad sense, to include specific preparatory behaviors, instrumental goal-seeking behaviors, as well as the spontaneous investigatory responses characteristic of motivational arousal. The term ‘consummatory phase’ is used in the spirit of Craig’s “phase II” of a motivated behavior “cycle” in which the “reception of the appeted stimulus” is followed by a “consummatory reaction in response to that stimulus” (see Craig 1918, p 101). The appetitive/consummatory distinction can refer to the temporal organization of behavior in which the consummatory reaction is that which terminates the behavioral sequence but can also refer to qualitative differences between the flexible behaviors leading up to goal attainment vs the relatively stereotyped action patterns observed during commerce with the goal (Craig 1918). Our usage conforms more to the latter theme. Hence, we use ‘consummatory phase’ to designate the period in which repetitive, relatively inflexible motor acts occur during actual contact with the food; chewing, licking, swallowing, etc. In the context of ingestive behavior (which involves actual consumption), the term ‘consumatory’ is sometimes preferred to ‘consummatory’ (see Smith 1995), but we will use the latter term simply to imply the generalizability of our proposed framework to other motivated behaviors, such as sexual behavior.
References
Aberman JE, Salamone JD (1999) Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience 92:545–552
Aberman JE, Ward SJ, Salamone JD (1998) Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav 61:341–348
Ahn S, Phillips AG (2002) Modulation by central and basolateral amygdalar nuclei of dopaminergic correlates of feeding to satiety in the rat nucleus accumbens and medial prefrontal cortex. J Neurosci 22:10958–10965
Anden NE, Carlsson A, Dahlstroem A, Fuxe K, Hillarp NA, Larsson K (1964) Demonstration and mapping out of nigro-neostriatal dopamine neurons. Life Sci 3:523–530
Arbuthnott GW, Ingham CA, Wickens JR (1998) Modulation by dopamine of rat corticostriatal input. Adv Pharmacol 42:733–736
Aston-Jones G, Bloom FE (1981) Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep–waking cycle. J Neurosci 1:876–886
Bakshi VP, Kelley AE (1991a) Dopaminergic regulation of feeding behavior: I. Differential effects of haloperidol microinfusion into three striatal subregions. Psychobiology 19:223–232
Bakshi VP, Kelley AE (1991b) Dopaminergic regulation of feeding behavior: II. Differential effects of amphetamine microinfusion into three striatal subregions. Psychobiology 19:233–242
Bakshi VP, Kelley AE (1993a) Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. J Pharmacol Exp Ther 265:1253–1260
Bakshi VP, Kelley AE (1993b) Striatal regulation of morphine-induced hyperphagia: an anatomical mapping study. Psychopharmacology (Berl) 111:207–214
Baldo BA, Sadeghian K, Basso AM, Kelley AE (2002) Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res 137:165–177
Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE (2004) Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. Eur J Neurosci 19:376–386
Baldwin AE, Holahan MR, Sadeghian K, Kelley AE (2000) N-methyl-d-aspartate receptor-dependent plasticity within a distributed corticostriatal network mediates appetitive instrumental learning. Behav Neurosci 114:84–98
Barbano MF, Cador M (2006) Differential regulation of the consummatory, motivational and anticipatory aspects of feeding behavior by dopaminergic and opioidergic drugs. Neuropsychopharmacology 31:1371–1381
Basso AM, Kelley AE (1999) Feeding induced by GABA(A) receptor stimulation within the nucleus accumbens shell: regional mapping and characterization of macronutrient and taste preference. Behav Neurosci 113:324–336
Bassareo V, Di Chiara G (1999) Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience 89:637–641
Bassareo V, De Luca MA, Di Chiara G (2002) Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. J Neurosci 22:4709–4719
Beninger RJ, Miller R (1998) Dopamine D1-like receptors and reward-related incentive learning. Neurosci Biobehav Rev 22:335–345
Berke JD, Hyman SE (2000) Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25:515–532
Berridge KC (2005) Espresso reward learning, hold the dopamine: theoretical comment on Robinson et al (2005) Behav Neurosci 119:336–341
Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28:309–369
Berridge KC, Venier IL, Robinson TE (1989) Taste reactivity analysis of 6-hydroxydopamine-induced aphagia: implications for arousal and anhedonia hypotheses of dopamine function. Behav Neurosci 103:36–45
Bindra D (1974) A motivational view of learning, performance, and behavior modification. Psychol Rev 81:199–213
Blackburn JR, Phillips AG, Fibiger HC (1987) Dopamine and preparatory behavior: I. Effects of pimozide. Behav Neurosci 101:352–360
Blackburn JR, Phillips AG, Jakubovic A, Fibiger HC (1989) Dopamine and preparatory behavior: II. A neurochemical analysis. Behav Neurosci 103:15–23
Blanchard RJ, Blanchard DC (1989) Antipredator defensive behaviors in a visible burrow system. J Comp Psychol 103:70–82
Blundell JE, Herberg LJ (1973) Effectiveness of lateral hypothalamic stimulation, arousal, and food deprivation in the initiation of hoarding behaviour in naive rats. Physiol Behav 10:763–767
Bodnar RJ, Lamonte N, Israel Y, Kandov Y, Ackerman TF, Khaimova E (2005) Reciprocal opioid–opioid interactions between the ventral tegmental area and nucleus accumbens regions in mediating mu agonist-induced feeding in rats. Peptides 26:621–629
Bradley MM, Codispoti M, Lang PJ (2006) A multi-process account of startle modulation during affective perception. Psychophysiology 43:486–497
Brody S, Rau H, Kohler F, Schupp H, Lutzenberger W, Birbaumer N (1994) Slow cortical potential biofeedback and the startle reflex. Biofeedback Self Regul 19:1–11
Buchwald NA, Horvath FE, Wyers EJ, Wakefield C (1964) Electroencephalogram rhythms correlated with milk reinforcement in cats. Nature 201:830–831
Burns LH, Everitt BJ, Kelley AE, Robbins TW (1994) Glutamate–dopamine interactions in the ventral striatum: role in locomotor activity and responding with conditioned reinforcement. Psychopharmacology (Berl) 115:516–528
Carlsson A, Dahlstroem A, Fuxe K, Lindqvist M (1965) Histochemical and biochemical detection of monoamine release from brain neurons. Life Sci 4:809–816
Cervantes M, De La Torre L, Beyer C (1975) Analysis of various factors involved in EEG synchronization during milk drinking in the cat. Brain Res 91:89–98
Clemente DC, Sterman MB, Wyrwicka W (1964) Post-reinforcement EEG synchronization during alimentary behavior. Electroencephalogr Clin Neurophysiol 16:355–365
Correa M, Carlson BB, Wisniecki A, Salamone JD (2002) Nucleus accumbens dopamine and work requirements on interval schedules. Behav Brain Res 137:179–187
Cousins MS, Wei W, Salamone JD (1994) Pharmacological characterization of performance on a concurrent lever pressing/feeding choice procedure: effects of dopamine antagonist, cholinomimetic, sedative and stimulant drugs. Psychopharmacology (Berl) 116:529–537
Craig W (1918) Appetites and aversions as constituents of instincts. Biol Bull 34:91–107
Cunningham ST, Kelley AE (1992) Opiate infusion into nucleus accumbens: contrasting effects on motor activity and responding for conditioned reward. Brain Res 588:104–114
Delfs JM, Schreiber L, Kelley AE (1990) Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat. J Neurosci 10:303–310
Dukas R (2002) Behavioural and ecological consequences of limited attention. Philos Trans R Soc Lond B Biol Sci 357:1539–1547
Ervin GN, Fink JS, Young RC, Smith GP (1977) Different behavioral responses to l-DOPA after anterolateral or posterolateral hypothalamic injections of 6-hydroxydopamine. Brain Res 132:507–520
Ettenberg A (1989) Dopamine, neuroleptics and reinforced behavior. Neurosci Biobehav Rev 13:105–111
Ettenberg A, Camp CH (1986a) Haloperidol induces a partial reinforcement extinction effect in rats: implications for a dopamine involvement in food reward. Pharmacol Biochem Behav 25:813–821
Ettenberg A, Camp CH (1986b) A partial reinforcement extinction effect in water-reinforced rats intermittently treated with haloperidol. Pharmacol Biochem Behav 25:1231–1235
Evans KR, Vaccarino FJ (1990) Amphetamine- and morphine-induced feeding: evidence for involvement of reward mechanisms. Neurosci Biobehav Rev 14:9–22
Everitt BJ (1990) Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev 14:217–232
Fibiger HC, Zis AP, McGeer EG (1973) Feeding and drinking deficits after 6-hydroxydopamine administration in the rat: similarities to the lateral hypothalamic syndrome. Brain Res 55:135–148
Fibiger HC, Carter DA, Phillips AG (1976) Decreased intracranial self-stimulation after neuroleptics or 6-hydroxydopamine: evidence for mediation by motor deficits rather than by reduced reward. Psychopharmacology (Berl) 47:21–27
Fiorillo CD, Tobler PN, Schultz W (2003) Discrete coding of reward probability and uncertainty by dopamine neurons. Science 299:1898–1902
Fouriezos G, Wise RA (1976) Pimozide-induced extinction of intracranial self-stimulation: response patterns rule out motor or performance deficits. Brain Res 103:377–380
Fouriezos G, Hansson P, Wise RA (1978) Neuroleptic-induced attenuation of brain stimulation reward in rats. J Comp Physiol Psychol 92:661–671
Fowler SC, Das S (1994) Haloperidol-induced decrements in force and duration of rats’ tongue movements during licking are attenuated by concomitant anticholinergic treatment. Pharmacol Biochem Behav 49:813–817
Fray PJ, Sahakian BJ, Robbins TW, Koob GF, Iversen SD (1980) An observational method for quantifying the behavioural effects of dopamine agonists: contrasting effects of d-amphetamine and apomorphine. Psychopharmacology (Berl) 69:253–259
Fuxe K, Hokfelt T, Olson L, Ungerstedt U (1977) Central monoaminergic pathways with emphasis on their relation to the so called ‘extrapyramidal motor system’. Pharmacol Ther B 3:169–210
Geyer MA, Russo PV, Segal DS, Kuczenski R (1987) Effects of apomorphine and amphetamine on patterns of locomotor and investigatory behavior in rats. Pharmacol Biochem Behav 28:393–399
Glickman SE, Schiff BB (1967) A biological theory of reinforcement. Psychol Rev 74:81–109
Gramling SE, Fowler SC, Collins KR (1984) Some effects of pimozide on nondeprived rats licking sucrose solutions in an anhedonia paradigm. Pharmacol Biochem Behav 21:617–624
Hackett JT, Marczynski TJ (1969) Postreinforcement electrocortical synchronization and enhancement of cortical photic evoked potentials during instrumentally conditioned appetitive behavior in the cat. Brain Res 15:447–464
Hajnal A, Smith GP, Norgren R (2004) Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol 286:R31–R37
Hanlon EC, Baldo BA, Sadeghian K, Kelley AE (2004) Increases in food intake or food-seeking behavior induced by GABAergic, opioid, or dopaminergic stimulation of the nucleus accumbens: is it hunger? Psychopharmacology (Berl) 172:241–247
Hernandez L, Hoebel BG (1988) Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol Behav 44:599–606
Hernandez PJ, Andrzejewski ME, Sadeghian K, Panksepp JB, Kelley AE (2005) AMPA/kainate, NMDA, and dopamine D1 receptor function in the nucleus accumbens core: a context-limited role in the encoding and consolidation of instrumental memory. Learn Mem 12:285–295
Hillarp NA, Fuxe K, Dahlstrom A (1966) Demonstration and mapping of central neurons containing dopamine, noradrenaline, and 5-hydroxytryptamine and their reactions to psychopharmaca. Pharmacol Rev 18:727–741
Hoebel BG, Teitelbaum P (1962) Hypothalamic control of feeding and self-stimulation. Science 135:375–377
Hollerman JR, Schultz W (1998) Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci 1:304–309
Hornykiewicz O (1966) Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev 18:925–964
Horvitz JC (2002) Dopamine gating of glutamatergic sensorimotor and incentive motivational input signals to the striatum. Behav Brain Res 137:65–74
Horvitz JC, Ettenberg A (1988) Haloperidol blocks the response-reinstating effects of food reward: a methodology for separating neuroleptic effects on reinforcement and motor processes. Pharmacol Biochem Behav 31:861–865
Ikemoto S, Panksepp J (1996) Dissociations between appetitive and consummatory responses by pharmacological manipulations of reward-relevant brain regions. Behav Neurosci 110:331–345
Ikemoto S, Panksepp J (1999) The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev 31:6–41
Imperato A, Angelucci L, Casolini P, Zocchi A, Puglisi-Allegra S (1992) Repeated stressful experiences differently affect limbic dopamine release during and following stress. Brain Res 577:194–199
Jacobs BL, Martin-Cora FJ, Fornal CA (2002) Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev 40:45–52
Jicha GA, Salamone JD (1991) Vacuous jaw movements and feeding deficits in rats with ventrolateral striatal dopamine depletion: possible relation to Parkinsonian symptoms. J Neurosci 11:3822–3829
Jones DL, Mogenson GJ (1979) Oral motor performance following central dopamine receptor blockade. Eur J Pharmacol 59:11–21
Kandov Y, Israel Y, Kest A, Dostova I, Verasammy J, Bernal SY, Kasselman L, Bodnar RJ (2006) GABA receptor subtype antagonists in the nucleus accumbens shell and ventral tegmental area differentially alter feeding responses induced by deprivation, glucoprivation and lipoprivation in rats. Brain Res 1082:86–97
Kelley AE (2004) Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron 44:161–179
Kelley AE, Delfs JM (1991) Dopamine and conditioned reinforcement. I. Differential effects of amphetamine microinjections into striatal subregions. Psychopharmacology (Berl) 103:187–196
Kelley AE, Stinus L (1985) Disappearance of hoarding behavior after 6-hydroxydopamine lesions of the mesolimbic dopamine neurons and its reinstatement with l-dopa. Behav Neurosci 99:531–545
Kelley AE, Swanson CJ (1997) Feeding induced by blockade of AMPA and kainate receptors within the ventral striatum: a microinfusion mapping study. Behav Brain Res 89:107–113
Kelley AE, Winnock M, Stinus L (1986) Amphetamine, apomorphine and investigatory behavior in the rat: analysis of the structure and pattern of responses. Psychopharmacology (Berl) 88:66–74
Kelley AE, Gauthier AM, Lang CG (1989) Amphetamine microinjections into distinct striatal subregions cause dissociable effects on motor and ingestive behavior. Behav Brain Res 35:27–39
Kelley AE, Bless EP, Swanson CJ (1996) Investigation of the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. J Pharmacol Exp Ther 278:1499–1507
Kelley AE, Smith-Roe SL, Holahan MR (1997) Response-reinforcement learning is dependent on N-methyl-d-aspartate receptor activation in the nucleus accumbens core. Proc Natl Acad Sci USA 94:12174–12179
Kelley AE, Baldo BA, Pratt WE (2005a) A proposed hypothalamic–thalamic–striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol 493:72–85
Kelley AE, Baldo BA, Pratt WE, Will MJ (2005b) Corticostriatal–hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav 86:773–795
Kerr JN, Wickens JR (2001) Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J Neurophysiol 85:117–124
Kiyatkin EA, Gratton A (1994) Electrochemical monitoring of extracellular dopamine in nucleus accumbens of rats lever-pressing for food. Brain Res 652:225–234
Koob GF, Le Moal M (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24:97–129
Koob GF, Riley SJ, Smith SC, Robbins TW (1978) Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat. J Comp Physiol Psychol 92:917–927
Krebs H, Macht M, Weyers P, Weijers HG, Janke W (1996) Effects of stressful noise on eating and non-eating behavior in rats. Appetite 26:193–202
Krebs H, Weyers P, Macht M, Weijers HG, Janke W (1997) Scanning behavior of rats during eating under stressful noise. Physiol Behav 62:151–154
Lehericy S, Benali H, Van de Moortele PF, Pelegrini-Issac M, Waechter T, Ugurbil K, Doyon J (2005) Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci USA 102:12566–12571
Liebman JM, Butcher LL (1973) Effects on self-stimulation behavior of drugs influencing dopaminergic neurotransmission mechanisms. Naunyn Schmiedebergs Arch Pharmacol 277:305–318
Lippa AS, Antelman SM, Fisher AE, Canfield DR (1973) Neurochemical mediation of reward: a significant role for dopamine? Pharmacol Biochem Behav 1:23–28
Lyon M, Robbins TW (1975) The action of central nervous system drugs: a general theory concerning amphetamine effects. In: Essman WB, Valzelli L (eds) Current developments in psychopharmacology. Spectrum, New York, pp 79–163
Maldonado-Irizarry CS, Swanson CJ, Kelley AE (1995) Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci 15:6779–6788
Margules DL, Olds J (1962) Identical “feeding” and “rewarding” systems in the lateral hypothalamus of rats. Science 135:374–375
Marshall JF, Richardson JS, Teitelbaum P (1974) Nigrostriatal bundle damage and the lateral hypothalamic syndrome. J Comp Physiol Psychol 87:808–830
McCullough LD, Salamone JD (1992) Involvement of nucleus accumbens dopamine in the motor activity induced by periodic food presentation: a microdialysis and behavioral study. Brain Res 592:29–36
McFarland K, Ettenberg A (1998) Haloperidol does not affect motivational processes in an operant runway model of food-seeking behavior. Behav Neurosci 112:630–635
Milner PM (1989) The discovery of self-stimulation and other stories. Neurosci Biobehav Rev 13:61–67
Mingote S, Weber SM, Ishiwari K, Correa M, Salamone JD (2005) Ratio and time requirements on operant schedules: effort-related effects of nucleus accumbens dopamine depletions. Eur J Neurosci 21:1749–1757
Mirenowicz J, Schultz W (1994) Importance of unpredictability for reward responses in primate dopamine neurons. J Neurophysiol 72:1024–1027
Mogenson GJ (1969) General and specific reinforcement systems for drinking behavior. Ann N Y Acad Sci 157:779–797
Mucha RF, Iversen SD (1986) Increased food intake after opioid microinjections into nucleus accumbens and ventral tegmental area of rat. Brain Res 397:214–224
Neill DB, Justice JBJ (1981) An hypothesis for a behavioral function of dopaminergic transmission in nucleus accumbens. In: Chronister RB, DeFrance JF (eds) The neurobiology of the nucleus accumbens. Haer Institute, Brunswick, pp 343–350
Nicola SM, Surmeier J, Malenka RC (2000) Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci 23:185–215
Nowend KL, Arizzi M, Carlson BB, Salamone JD (2001) D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav 69:373–382
O’Donnell P (2003) Dopamine gating of forebrain neural ensembles. Eur J Neurosci 17:429–435
Olds J, Milner P (1954) Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol 47:419–427
Olds J, Allan WS, Briese E (1971) Differentiation of hypothalamic drive and reward centers. Am J Physiol 221:368–375
Onuki Y, Makino J (2005) Food-carrying behavior increased under risk-approaching signal in rats (Rattus norvegicus). Physiol Behav 84:141–145
Paulus MP, Geyer MA, Gold LH, Mandell AJ (1990) Application of entropy measures derived from the ergodic theory of dynamical systems to rat locomotor behavior. Proc Natl Acad Sci USA 87:723–727
Paulus MP, Callaway CW, Geyer MA (1993) Quantitative assessment of the microstructure of rat behavior: II. Distinctive effects of dopamine releasers and uptake inhibitors. Psychopharmacology (Berl) 113:187–198
Pecina S, Berridge KC (2000) Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Res 863:71–86
Pecina S, Berridge KC (2005) Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci 25:11777–11786
Pfaus JG, Phillips AG (1991) Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav Neurosci 105:727–743
Phillips AG, Atkinson LJ, Blackburn JR, Blaha CD (1993) Increased extracellular dopamine in the nucleus accumbens of the rat elicited by a conditional stimulus for food: an electrochemical study. Can J Physiol Pharmacol 71:387–393
Phillips GD, Robbins TW, Everitt BJ (1994) Mesoaccumbens dopamine–opiate interactions in the control over behaviour by a conditioned reinforcer. Psychopharmacology (Berl) 114:345–359
Pijnenburg AJ, Honig WM, Van der Heyden JA, Van Rossum JM (1976) Effects of chemical stimulation of the mesolimbic dopamine system upon locomotor activity. Eur J Pharmacol 35:45–58
Radhakishun FS, van Ree JM, Westerink BH (1988) Scheduled eating increases dopamine release in the nucleus accumbens of food-deprived rats as assessed with on-line brain dialysis. Neurosci Lett 85:351–356
Reynolds SM, Berridge KC (2001) Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci 21:3261–3270
Reynolds SM, Berridge KC (2002) Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. J Neurosci 22:7308–7320
Richardson NR, Gratton A (1996) Behavior-relevant changes in nucleus accumbens dopamine transmission elicited by food reinforcement: an electrochemical study in rat. J Neurosci 16:8160–8169
Robbins TW, Everitt BJ (2002) Limbic–striatal memory systems and drug addiction. Neurobiol Learn Mem 78:625–636
Robinson S, Sandstrom SM, Denenberg VH, Palmiter RD (2005) Distinguishing whether dopamine regulates liking, wanting, and/or learning about rewards. Behav Neurosci 119:5–15
Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM (2004) Dopamine operates as a subsecond modulator of food seeking. J Neurosci 24:1265–1271
Salamone JD, Correa M (2002) Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res 137:3–25
Salamone JD, Mahan K, Rogers S (1993) Ventrolateral striatal dopamine depletions impair feeding and food handling in rats. Pharmacol Biochem Behav 44:605–610
Salamone JD, Cousins MS, Bucher S (1994a) Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res 65:221–229
Salamone JD, Cousins MS, McCullough LD, Carriero DL, Berkowitz RJ (1994b) Nucleus accumbens dopamine release increases during instrumental lever pressing for food but not free food consumption. Pharmacol Biochem Behav 49:25–31
Salamone JD, Cousins MS, Snyder BJ (1997) Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev 21:341–359
Salamone JD, Wisniecki A, Carlson BB, Correa M (2001) Nucleus accumbens dopamine depletions make animals highly sensitive to high fixed ratio requirements but do not impair primary food reinforcement. Neuroscience 105:863–870
Schmitt B, Marshall L, Nitsche M, Hallschmid M, Eulitz C, Born J (2000) Slow cortical DC-potential responses to sweet and bitter tastes in humans. Physiol Behav 71:581–587
Schneider LH, Davis JD, Watson CA, Smith GP (1990) Similar effect of raclopride and reduced sucrose concentration on the microstructure of sucrose sham feeding. Eur J Pharmacol 186:61–70
Schultz W (2002) Getting formal with dopamine and reward. Neuron 36:241–263
Schultz W, Apicella P, Ljungberg T (1993) Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci 13:900–913
Schupp HT, Lutzenberger W, Rau H, Birbaumer N (1994) Positive shifts of event-related potentials: a state of cortical disfacilitation as reflected by the startle reflex probe. Electroencephalogr Clin Neurophysiol 90:135–144
Schwartzbaum JS, Kreinick CJ, Mello WF (1972) Cortical evoked potentials and synchronization of electrocortical activity during consummatory behavior in rats. Brain Res 36:171–182
Smith GP (1995) Dopamine and food reward. Prog Psychobiol Physiol Psychol 16:83–144
Smith GP (2004) Accumbens dopamine mediates the rewarding effect of orosensory stimulation by sucrose. Appetite 43:11–13
Smith-Roe SL, Kelley AE (2000) Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci 20:7737–7742
Stellar E (1954) The physiology of motivation. Psychol Rev 61:5–22
Stephan FK, Valenstein ES, Zucker I (1971) Copulation and eating during electrical stimulation of the rat hypothalamus. Physiol Behav 7:587–593
Sterman MB, Wyrwicka W (1967) EEG correlates of sleep: evidence for separate forebrain substrates. Brain Res 6:143–163
Stratford TR, Kelley AE (1997) GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci 17:4434–4440
Stratford TR, Kelley AE (1999) Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci 19:11040–11048
Stratford TR, Swanson CJ, Kelley A (1998) Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behav Brain Res 93:43–50
Swanson LW (2000) Cerebral hemisphere regulation of motivated behavior. Brain Res 886:113–164
Taha SA, Fields HL (2005) Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. J Neurosci 25:1193–1202
Tidey JW, Miczek KA (1996) Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res 721:140–149
Toates F (1986) Motivational systems. Cambridge, MA, Cambridge University Press
Tombaugh TN, Tombaugh J, Anisman H (1979) Effects of dopamine receptor blockade on alimentary behaviors: home cage food consumption, magazine training, operant acquisition, and performance. Psychopharmacology (Berl) 66:219–225
Tyrka A, Smith GP (1991) Potency of SCH 23390 for decreasing sucrose intake in rat pups depends on mode of ingestion. Pharmacol Biochem Behav 39:955–961
Tyrka A, Gayle C, Smith GP (1992) Raclopride decreases sucrose intake of rat pups in independent ingestion tests. Pharmacol Biochem Behav 43:863–869
Ungerstedt U (1970) Is interruption of the nigro-striatal dopamine system producing the “lateral hypothalamus syndrome”? Acta Physiol Scand 80:35A–36A
Ungerstedt U, Arbuthnott GW (1970) Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res 24:485–493
Ungerstedt U, Ljungberg T (1974) Central dopamine neurons and sensory processing. J Psychiatr Res 11:149–150
Valenstein ES, Cox VC (1970) Influence of hunger, thirst, and previous experience in the test chamber on stimulus-bound eating and drinking. J Comp Physiol Psychol 70:189–199
van den Bos R, Cools AR (1989) The involvement of the nucleus accumbens in the ability of rats to switch to cue-directed behaviours. Life Sci 44:1697–1704
Vanderwolf CH (1975) Neocortical and hippocampal activation relation to behavior: effects of atropine, eserine, phenothiazines, and amphetamine. J Comp Physiol Psychol 88:300–323
West AR, Floresco SB, Charara A, Rosenkranz JA, Grace AA (2003) Electrophysiological interactions between striatal glutamatergic and dopaminergic systems. Ann N Y Acad Sci 1003:53–74
White NM (1989) Reward or reinforcement: what’s the difference? Neurosci Biobehav Rev 13:181–186
Wickens JR, Reynolds JN, Hyland BI (2003) Neural mechanisms of reward-related motor learning. Curr Opin Neurobiol 13:685–690
Wilson C, Nomikos GG, Collu M, Fibiger HC (1995) Dopaminergic correlates of motivated behavior: importance of drive. J Neurosci 15:5169–178
Wise RA (1982) Neuroleptics and operant behavior: the anhedonia hypothesis. Behav Brain Sci 5:39–87
Wise RA (1985) The anhedonia hypothesis: Mark III. Behav Brain Sci 8:178–186
Wise RA (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5:483–494
Wise RA, Colle LM (1984) Pimozide attenuates free feeding: best scores analysis reveals a motivational deficit. Psychopharmacology (Berl) 84:446–451
Wise RA, Raptis L (1986) Effects of naloxone and pimozide on initiation and maintenance measures of free feeding. Brain Res 368:62–68
Wise RA, Spindler J, deWit H, Gerberg GJ (1978a) Neuroleptic-induced “anhedonia” in rats: pimozide blocks reward quality of food. Science 201:262–264
Wise RA, Spindler J, Legault L (1978b) Major attenuation of food reward with performance-sparing doses of pimozide in the rat. Can J Psychol 32:77–85
Wolterink G, Phillips G, Cador M, Donselaar-Wolterink I, Robbins TW, Everitt BJ (1993) Relative roles of ventral striatal D1 and D2 dopamine receptors in responding with conditioned reinforcement. Psychopharmacology (Berl) 110:355–364
Wyvell CL, Berridge KC (2000) Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci 20:8122–8130
Yokel RA, Wise RA (1975) Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science 187:547–549
Zhang M, Kelley AE (2000) Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and Fos expression. Neuroscience 99:267–277
Zhang M, Kelley AE (2002) Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 159:415–423
Zhang M, Gosnell BA, Kelley AE (1998) Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther 285:908–914
Zhang M, Balmadrid C, Kelley AE (2003) Nucleus accumbens opioid, GABAergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci 117:202–211
Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR (2003) Peptides that regulate food intake: appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol 284:R1436–R1444
Zis AP, Fibiger HC (1975) Neuroleptic-induced deficits in food and water regulation: similarities to the lateral hypothalamic syndrome. Psychopharmacologia 43:63–68
Acknowledgements
This work was supported by National Institute on Drug Abuse grant DA 09311 (A.E.K.) and National Institute on Mental Health grant MH 74723 (B.A.B).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baldo, B.A., Kelley, A.E. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology 191, 439–459 (2007). https://doi.org/10.1007/s00213-007-0741-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0741-z