Abstract
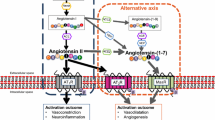
Omapatrilat (OMA), which simultaneously inhibits the angiotensin-converting enzyme (ACE) and the neutral endopeptidase (neprilysin (NEP)), is widely used in experimental protocols related to hypertension and heart failure. The penetration of OMA across the blood–brain barrier (BBB) and the effects of ACE/NEP inhibition on the recovery from ischaemic stroke have not yet been investigated. Angiotensin (Ang) I injected intracerebroventricularly (ICV) or intravenously (IV) is converted to Ang II by ACE and induces an immediate increase in blood pressure. The pressor responses to OMA administered ICV, orally or IV were studied in male Wistar rats instrumented with an ICV and arterial and venous catheters. OMA infused ICV rapidly appeared in the systemic circulation and more effectively attenuated the systemic than the central pressor responses to Ang I. OMA administered orally (5, 25, 100 μmol/kg body weight) or IV (0.5, 1, 5, 25 μmol/kg body weight) completely abolished increases in blood pressure to IV Ang I up to 2 h after treatment. The pressor responses to ICV Ang I were not altered, indicating that systemically administered OMA does not cross the BBB. To study the effects of ACE and NEP inhibition in the brain on the recovery from ischaemic stroke, OMA was infused ICV over a 5-day period before and 24 h after the occlusion of the middle cerebral artery (MCAO) for 90 min. ICV application of OMA had no effect on infarction volume and marginally improved neurological outcome. We demonstrate for the first time that simultaneous inhibition of ACE and NEP in the brain tissue does not alter the recovery from ischaemic stroke.





Similar content being viewed by others
References
Bains JS, Potyok A, Ferguson AV (1992) Angiotensin II actions in paraventricular nucleus: functional evidence for neurotransmitter role in efferents originating in subfornical organ. Brain Res 599(2):223–229
Birner C, Ulucan C, Bratfisch M, Götz T, Dietl A, Schweda F, Riegger GA, Luchner A (2012) Antihypertrophic effects of combined inhibition of the renin-angiotensin system (RAS) and neutral endopeptidase (NEP) in progressive, tachycardia-induced experimental heart failure. Naunyn Schmiedeberg's Arch Pharmacol 385(11):1117–1125
Burnett JC Jr (1999) Vasopeptidase inhibition: a new concept in blood pressure management. J Hypertens Suppl 17(1):S37–S43
Chai SY, Mendelsohn FA, Paxinos G (1987) Angiotensin converting enzyme in rat brain visualized by quantitative in vitro autoradiography. Neuroscience 20(2):615–627
Cuculi F, Erne P (2011) Combined neutral endopeptidase inhibitors. Expert Opin Investig Drugs 20(4):457–463
Culman J, Klee S, Ohlendorf C, Unger T (1997) Effect of tachykinin receptor inhibition in the brain on cardiovascular and behavioral responses to stress. J Pharmacol Exp Ther 280(1):238–246
Dai WJ, Funk A, Herdegen T, Unger T, Culman J (1999) Blockade of central angiotensin AT(1) receptors improves neurological outcome and reduces expression of AP-1 transcription factors after focal brain ischemia in rats. Stroke 30(11):2391–2398, discussion 2398–2399
Dalzell JR, Seed A, Berry C, Whelan CJ, Petrie MC, Padmanabhan N, Clarke A, Biggerstaff F, Hillier C, McMurray JJ (2014) Effects of neutral endopeptidase (neprilysin) inhibition on the response to other vasoactive peptides in small human resistance arteries: studies with thiorphan and omapatrilat. Cardiovasc Ther 32(1):13–18
Ding-Zhou L, Margaill I, Palmier B, Pruneau D, Plotkine M, Marchand-Verrecchia C (2003) LF 16-0687 Ms, a bradykinin B2 receptor antagonist, reduces ischemic brain injury in a murine model of transient focal cerebral ischemia. Br J Pharmacol 139(8):1539–1547
Engelhardt B, Sorokin L (2009) The blood–brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol 31(4):497–511
Garcia JH, Wagner S, Liu KF, Hu XJ (1995) Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 26(4):627–634, discussion 635
Gohlke P, Schölkens BA ACE inhibitors: pharmacology (2004) In: Angiotensin, Handb. Exp.Pharm.163/II T. Unger, BA Schölkens (Eds), Springer-Verlag, pp. 375–413
Gohlke P, Schölkens B, Henning R, Urbach H, Unger T (1989) Inhibition of converting enzyme in brain tissue and cerebrospinal fluid of rats following chronic oral treatment with the converting enzyme inhibitors ramipril and Hoe 288. J Cardiovasc Pharmacol 14(Suppl 4):S32–S36
Gröger M, Lebesgue D, Pruneau D, Relton J, Kim SW, Nussberger J, Plesnila N (2005) Release of bradykinin and expression of kinin B2 receptors in the brain: role for cell death and brain edema formation after focal cerebral ischemia in mice. J Cereb Blood Flow Metab 25(8):978–989
Haack D, Möhring J (1978) Vasopressin-mediated blood pressure response to intraventricular injection of angiotensin II in the rat. Pflugers Arch 373(2):167–173
Hogarty DC, Speakman EA, Puig V, Phillips MI (1992) The role of angiotensin, AT1 and AT2 receptors in the pressor, drinking and vasopressin responses to central angiotensin. Brain Res 586(2):289–294
Honda K, Negoro H, Dyball RE, Higuchi T, Takano S (1990) The osmoreceptor complex in the rat: evidence for interactions between the supraoptic and other diencephalic nuclei. J Physiol 431:225–241
Iwata N, Higuchi M, Saido TC (2005) Metabolism of amyloid-beta peptide and Alzheimer’s disease. Pharmacol Ther 108(2):129–148
Johanson CE, Stopa EG, McMillan PN (2011) The blood-cerebrospinal fluid barrier: structure and functional significance. In: The blood–brain and other neural barriers: reviews and protocols, Nag S (ed), Methods in Molecular Biology, Vol. 686, Humana Press, Springer Verlag, pp. 101–131
Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E (2004) Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens 17(2):103–111
Krisch B, Leonhardt H, Buchheim W (1978) The functional and structural border between the CSF- and blood-milieu in the circumventricular organs (organum vasculosum laminae terminalis, subfornical organ, area postrema) of the rat. Cell Tissue Res 195(3):485–497
Krisch B, Leonhardt H, Oksche A (1987) Compartments in the organum vasculosum laminae terminalis of the rat and their delineation against the outer cerebrospinal fluid-containing space. Cell Tissue Res 250(2):331–347
Kubota E, Dean RG, Hubner RA, Casley DJ, Johnston CI, Burrell LM (2003) Differential tissue and enzyme inhibitory effects of the vasopeptidase inhibitor omapatrilat in the rat. Clin Sci (Lond) 105(3):339–345
Lapchak PA, Zhang JH, Noble-Haeusslein LJ (2013) RIGOR guidelines: escalating STAIR and STEPS for effective translational research. Transl Stroke Res 4(3):279–285
Li J, Culman J, Hörtnagl H, Zhao Y, Gerova N, Timm M, Blume A, Zimmermann M, Seidel K, Dirnagl U, Unger T (2005) Angiotensin AT2 receptor protects against cerebral ischemia-induced neuronal injury. FASEB J 19(6):617–619
Lou M, Blume A, Zhao Y, Gohlke P, Deuschl G, Herdegen T, Culman J (2004) Sustained blockade of brain AT1 receptors before and after focal cerebral ischemia alleviates neurologic deficits and reduces neuronal injury, apoptosis, and inflammatory responses in the rat. J Cereb Blood Flow Metab 24(5):536–547
Mangiafico S, Costello-Boerrigter LC, Andersen IA, Cataliotti A, Burnett JC Jr (2013) Neutral endopeptidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeutics. Eur Heart J 34(12):886–893
Mendelsohn FA, Quirion R, Saavedra JM, Aguilera G, Catt KJ (1984) Autoradiographic localization of angiotensin II receptors in rat brain. Proc Natl Acad Sci U S A 81(5):1575–1579
Min LJ, Mogi M, Tsukuda K, Jing F, Ohshima K, Nakaoka H, Kan-No H, Wang XL, Chisaka T, Bai HY, Iwanami J, Horiuchi M (2014) Direct stimulation of angiotensin II type 2 receptor initiated after stroke ameliorates ischemic brain damage. Am J Hypertens 27(8):1036–1044
Murtha LA, Yang Q, Parsons MW, Levi CR, Beard DJ, Spratt NJ, McLeod DD (2014) Cerebrospinal fluid is drained primarily via the spinal canal and olfactory route in young and aged spontaneously hypertensive rats. Fluids Barriers CNS 11:12
Oldfield BJ, Hards DK, McKinley MJ (1991) Projections from the subfornical organ to the supraoptic nucleus in the rat: ultrastructural identification of an interposed synapse in the median preoptic nucleus using a combination of neuronal tracers. Brain Res 558(1):13–19
Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD (2006) Blood–brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol 1(3):223–236
Rashid M, Wangler NJ, Yang L, Shah K, Arumugam TV, Abbruscato TJ, Karamyan VT (2014) Functional up-regulation of endopeptidase neurolysin during post-acute and early recovery phases of experimental stroke in mouse brain. J Neurochem 129(1):179–189
Robl JA, Sun CQ, Stevenson J, Ryono DE, Simpkins LM, Cimarusti MP, Dejneka T, Slusarchyk WA, Chao S, Stratton L, Misra RN, Bednarz MS, Asaad MM, Cheung HS, Abboa-Offei BE, Smith PL, Mathers PD, Fox M, Schaeffer TR, Seymour AA, Trippodo NC (1997) Dual metalloprotease inhibitors: mercaptoacetyl-based fused heterocyclic dipeptide mimetics as inhibitors of angiotensin-converting enzyme and neutral endopeptidase. J Med Chem 40(11):1570–1577
Rosenberg GA (2012) Neurological diseases in relation to the blood–brain barrier. J Cereb Blood Flow Metab 32(7):1139–1151
Saavedra JM, Chevillard C (1982) Angiotensin-converting enzyme is present in the subfornical organ and other circumventricular organs of the rat. Neurosci Lett 29(2):123–127
Sobey CG (2003) Bradykinin B2 receptor antagonism: a new direction for acute stroke therapy? Br J Pharmacol 139(8):1369–1371
Speake T, Whitwell C, Kajita H, Majid A, Brown PD (2001) Mechanisms of CSF secretion by the choroid plexus. Microsc Res Tech 52(1):49–59
Turner RJ, Helps SC, Thornton E, Vink R (2011) A substance P antagonist improves outcome when administered 4 h after onset of ischaemic stroke. Brain Res 1393:84–90
Unger T, Badoer E, Ganten D, Lang RE, Rettig R (1988) Brain angiotensin: pathways and pharmacology. Circulation 77(6 Pt 2):I40–I54
von Lueder TG, Atar D, Krum H (2014) Current role of neprilysin inhibitors in hypertension and heart failure. Pharmacol Ther 144(1):41–49
Yu Z, Cheng G, Huang X, Li K, Cao X (1997) Neurokinin-1 receptor antagonist SR140333: a novel type of drug to treat cerebral ischemia. Neuroreport 8(9–10):2117–2119
Zhao Y, Patzer A, Herdegen T, Gohlke P, Culman J (2006) Activation of cerebral peroxisome proliferator-activated receptors gamma promotes neuroprotection by attenuation of neuronal cyclooxygenase-2 overexpression after focal cerebral ischemia in rats. FASEB J 20(8):1162–1175
Zheng W, Monnot AD (2012) Regulation of brain iron and copper homeostasis by brain barrier systems: implication in neurodegenerative diseases. Pharmacol Ther 133(2):177–188
Zuhayra M, Zhao Y, von Forstner C, Henze E, Gohlke P, Culman J, Lützen U (2011) Activation of cerebral peroxisome proliferator-activated receptors γ (PPARγ) reduces neuronal damage in the substantia nigra after transient focal cerebral ischaemia in the rat. Neuropathol Appl Neurobiol 37(7):738–752
Acknowledgments
The authors would like to thank laboratory technicians Mr. Jan Brdon and Ms. B. Schwarten for the implantation of osmotic minipumps.
Compliance with Ethical Standards
This research was not supported by specific grant from any funding agency in the public, commercial or not-for-profit sectors. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmedt auf der Günne, W., Zhao, Y., Hedderich, J. et al. Omapatrilat: penetration across the blood–brain barrier and effects on ischaemic stroke in rats. Naunyn-Schmiedeberg's Arch Pharmacol 388, 939–951 (2015). https://doi.org/10.1007/s00210-015-1126-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-015-1126-1