Abstract
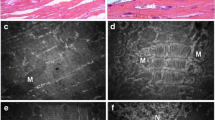
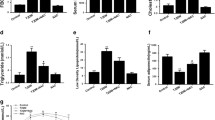
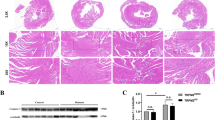
Diabetic cardiomyopathy (DC) is a unique disease frequently complicated to diabetes mellitus, manifesting endoplasmic reticulum (ER) stress and depressed calcium-handling proteins. We hypothesized that the abnormal FKBP12.6, SERCA2a, and CASQ2 are consequent to ER stress and apoptosis that are likely due to an entity of inflammation. These abnormalities may be attributed to reactive oxygen species genesis from activated NADPH oxidase which could respond to argirein (AR) through its anti-inflammatory activity. Sprague Dawley rats were randomly divided into six groups. Except the normal group, rats were injected with streptozotocin (STZ; 60 mg/kg, i.p.) once. During weeks 5 to 8 following STZ injection, rats were treated (in milligrams per kilogram per day, i.g.) with aminoguanidine (AMG, 100; an inducible nitric oxide synthase and AGEs inhibitor) or three doses of AR (50, 100, and 200). FKBP12.6, SERCA2a, and CASQ2 and ER stress chaperones Bip and PERK and apoptotic molecules were monitored in vivo and in vitro. Impaired cardiac performance and downregulated FKBP12.6, SERCA2a, and CASQ2 were significant in DC in vivo, and abnormal calcium-handling proteins were also found in high-glucose-incubated myocytes in vitro. ER stress manifested by upregulated Bip and PERK was predominant in association with DNA ladder and upregulated Bax and downregulated BCL-2 in vivo and in vitro. AR is effective to attenuate these abnormalities compared to AMG. Diabetic myocardium has inflammatory entity expressed as ER stress contributing to downregulated calcium-handling proteins. AR has potential in managing DC through attenuating depressed calcium-handling proteins, activated ER stress, and apoptosis in the myocardium.






Similar content being viewed by others
Abbreviations
- AGEs:
-
Advanced glycation end products
- AMG:
-
Aminoguanidine
- AR:
-
Argirein
- ATF-4:
-
Activating transcription factor-4
- ATF-6:
-
Activating transcription factor-6
- AT1:
-
Angiotensin 1 receptors
- Bip:
-
Immunoglobulin heavy chain binding protein
- Bax:
-
BCL-2 associated X protein
- BCL-2:
-
B cell CLL/lymphoma
- CASQ2:
-
Calsequestrin 2
- CHOP:
-
C/EBP homologous protein
- DC:
-
Diabetic cardiomyopathy
- DM:
-
Diabetes mellitus
- ER stress:
-
Endoplasmic reticulum stress
- ER:
-
Endoplasmic reticulum
- ETA :
-
Endothelin receptor A
- FKBP12.6:
-
FK506 binding protein 12.6
- iNOS:
-
Inducible nitric oxide synthase
- IRE1:
-
Inositol-requiring enzyme-1
- PERK:
-
PKR-like eukaryotic initiation factor 2α kinase
- ROS:
-
Reactive oxygen species
- RyR2:
-
Ryanodine receptor subtype 2
- STZ:
-
Streptozotocin
- SERCA2a:
-
Sarco/endoplasmic reticulum calcium ATPase 2a
- SD:
-
Sprague Dawley
- UPR:
-
Unfolded protein response
References
Aksakal E, Akaras N, Kurt M, Tanboga IH, Halici Z, Odabasoglu F, Bakirci EM, Unal B (2011) The role of oxidative stress in diabetic cardiomyopathy: an experimental study. Eur Rev Med Pharmacol Sci 15(11):1241–1246
Cai L, Wang Y, Zhou G, Chen T, Song Y, Li X, Kang YJ (2006) Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol 48(8):1688–1697
Cheng YS, Dai DZ, Ji H, Zhang Q, Dai Y (2011) Sildenafil and FDP-Sr attenuate diabetic cardiomyopathy by suppressing abnormal expression of myocardial CASQ2, FKBP12.6, and SERCA2a in rats. Acta Pharmacol Sin 32(4):441–448
Cheng YS, Tang YQ, Dai DZ, Dai Y (2012) AQP4 knockout mice manifest abnormal expressions of calcium handling proteins possibly due to exacerbating pro-inflammatory factors in the heart. Biochem Pharmacol 83(1):97–105
Cong XD, Ding MJ, Dai DZ, Wu Y, Zhang Y, Dai Y (2012a) ER stress, p66shc, and p-Akt/Akt mediate adjuvant-induced inflammation, which is blunted by argirein, a supermolecule and rhein in rats. Inflammation 35(3):1031–1040
Cong XD, Fu PR, Dai DZ, Zhang Y, Dai Y (2012b) Pharmacokinetic behavior of argirein, derived from rhein, is characterized as slow release and prolonged T1/2 of rhein in rats. Eur J Pharm Sci 46(5):468–474
Cong XD, Wu Y, Dai DZ, Ding MJ, Zhang Y, Dai Y (2012c) Activation of AQP4, p66Shc and endoplasmic reticulum stress is involved in inflammation by carrageenan and is suppressed by argirein, a derivative of rhein. J Pharm Pharmacol 64(8):1138–1145
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116(2):205–219
Duncan JG (2011) Mitochondrial dysfunction in diabetic cardiomyopathy. Biochim Biophys Acta 1813(7):1351–1359
Fu ZQ, Li XY, Lu XC, Mi YF, Liu T, Ye WH (2012) Ameliorated stress related proteins are associated with improved cardiac function by sarcoplasmic reticulum calcium ATPase gene transfer in heart failure. J Geriatr Cardiol 9(3):269–277
Gan R, Hu G, Zhao Y, Li H, Jin Z, Ren H, Dong S, Zhong X, Yang B, Xu C, Lu F, Zhang W (2012) Post-conditioning protecting rat cardiomyocytes from apoptosis via attenuating calcium-sensing receptor-induced endo(sarco)plasmic reticulum stress. Mol Cell Biochem 361(1–2):123–134
Gao Q, Qin WS, Jia ZH, Zheng JM, Zeng CH, Li LS, Liu ZH (2010) Rhein improves renal lesion and ameliorates dyslipidemia in db/db mice with diabetic nephropathy. Planta Med 76(1):27–33
George I, Sabbah HN, Xu K, Wang N, Wang J (2011) Beta-adrenergic receptor blockade reduces endoplasmic reticulum stress and normalizes calcium handling in a coronary embolization model of heart failure in canines. Cardiovasc Res 91(3):447–455
Glembotski CC (2008) The role of the unfolded protein response in the heart. J Mol Cell Cardiol 44(3):453–459
Groenendyk J, Sreenivasaiah PK, do Kim H, Agellon LB, Michalak M (2010) Biology of endoplasmic reticulum stress in the heart. Circ Res 107(10):1185–1197
Gullestad L, Ueland T, Vinge LE, Finsen A, Yndestad A, Aukrust P (2012) Inflammatory cytokines in heart failure: mediators and markers. Cardiology 122(1):23–35
Hu C, Cong XD, Dai DZ, Zhang Y, Zhang GL, Dai Y (2011a) Argirein alleviates diabetic nephropathy through attenuating NADPH oxidase, Cx43, and PERK in renal tissue. Naunyn Schmiedebergs Arch Pharmacol 383(3):309–319
Hu ST, Liu GS, Shen YF, Wang YL, Tang Y, Yang YJ (2011b) Defective Ca(2+) handling proteins regulation during heart failure. Physiol Res 60(1):27–37
Kain V, Kumar S, Sitasawad SL (2011) Azelnidipine prevents cardiac dysfunction in streptozotocin-diabetic rats by reducing intracellular calcium accumulation, oxidative stress and apoptosis. Cardiovasc Diabetol 10:97
Kranstuber AL, Del Rio C, Biesiadecki BJ, Hamlin RL, Ottobre J, Gyorke S, Lacombe VA (2012) Advanced glycation end product cross-link breaker attenuates diabetes-induced cardiac dysfunction by improving sarcoplasmic reticulum calcium handling. Front Physiol 3:292
Li J, Zhu H, Shen E, Wan L, Arnold JM, Peng T (2010) Deficiency of rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes 59(8):2033–2042
Li N, Jia N, Dai DZ, Dai Y (2008a) Endothelin receptor antagonist CPU0213 and vitamin E reverse downregulation of FKBP12.6 and SERCA2a: a role of hyperphosphorylation of PKCepsilon. Eur J Pharmacol 591(1–3):211–218
Li Z, Zhang T, Dai H, Liu G, Wang H, Sun Y, Zhang Y, Ge Z (2007) Involvement of endoplasmic reticulum stress in myocardial apoptosis of streptozocin-induced diabetic rats. J Clin Biochem Nutr 41(1):58–67
Li Z, Zhang T, Dai H, Liu G, Wang H, Sun Y, Zhang Y, Ge Z (2008b) Endoplasmic reticulum stress is involved in myocardial apoptosis of streptozocin-induced diabetic rats. J Endocrinol 196(3):565–572
Liu GL, Yu F, Dai DZ, Zhang GL, Zhang C, Dai Y (2012) Endoplasmic reticulum stress mediating downregulated StAR and 3-beta-HSD and low plasma testosterone caused by hypoxia is attenuated by CPU86017-RS and nifedipine. J Biomed Sci 19:4
Liu HR, Tang XY, Dai DZ, Dai Y (2008) Ethanol extracts of Rehmannia complex (Di Huang) containing no Corni fructus improve early diabetic nephropathy by combining suppression on the ET-ROS axis with modulate hypoglycemic effect in rats. J Ethnopharmacol 118(3):466–472
Liu XH, Zhang ZY, Andersson KB, Husberg C, Enger UH, Raeder MG, Christensen G, Louch WE (2011) Cardiomyocyte-specific disruption of Serca2 in adult mice causes sarco(endo)plasmic reticulum stress and apoptosis. Cell Calcium 49(4):201–207
Minamino T, Komuro I, Kitakaze M (2010) Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res 107(9):1071–1082
Montagnani M (2008) Diabetic cardiomyopathy: how much does it depend on AGE? Br J Pharmacol 154(4):725–726
Na T, Dai DZ, Tang XY, Dai Y (2007) Upregulation of leptin pathway correlates with abnormal expression of SERCA2a, phospholamban and the endothelin pathway in heart failure and reversal by CPU86017. Naunyn Schmiedebergs Arch Pharmacol 375(1):39–49
Ni L, Zhou C, Duan Q, Lv J, Fu X, Xia Y, Wang DW (2011) Beta-AR blockers suppresses ER stress in cardiac hypertrophy and heart failure. PLoS One 6(11):e27294
Niu J, Azfer A, Rogers LM, Wang X, Kolattukudy PE (2007) Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc Res 73(3):549–559
Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A (2007) ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129(7):1337–1349
Qi MY, Liu HR, Dai DZ, Li N, Dai Y (2008) Total triterpene acids, active ingredients from Fructus Corni, attenuate diabetic cardiomyopathy by normalizing ET pathway and expression of FKBP12.6 and SERCA2a in streptozotocin-rats. J Pharm Pharmacol 60(12):1687–1694
Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ (2008) Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest 118(10):3378–3389
Seferović PM, Milinković I, Ristić AD, Seferović Mitrović JP, Lalić K, Jotić A, Kanjuh V, Lalić N, Maisch B (2012) Diabetic cardiomyopathy: ongoing controversies in 2012. Herz 37(8):880–886
Szegezdi E, Logue SE, Gorman AM, Samali A (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO reports 7(9):880–885
Tillquist MN, Maddox TM (2012) Update on diabetic cardiomyopathy: inches forward, miles to go. Curr Diab Rep 12(3):305–313
Toth A, Nickson P, Mandl A, Bannister ML, Toth K, Erhardt P (2007) Endoplasmic reticulum stress as a novel therapeutic target in heart diseases. Cardiovasc Hematol Disord Drug Targets 7(3):205–218
Xu J, Wang G, Wang Y, Liu Q, Xu W, Tan Y, Cai L (2009) Diabetes- and angiotensin II-induced cardiac endoplasmic reticulum stress and cell death: metallothionein protection. J Cell Mol Med 13(8A):1499–1512
Xu J, Zhou Q, Xu W, Cai L (2012) Endoplasmic reticulum stress and diabetic cardiomyopathy. Exp Diabetes Res 2012:827971
Zhang GL, Dai DZ, Xi T, Cong XD, Zhang Y, Dai Y (2011) Isoproterenol-induced FKBP12.6/12 downregulation is modulated by ETA and ETB receptors and reversed by argirhein, a derivative of rhein. Acta Pharmacol Sin 32(2):223–229
Zhang X, Chen C (2012) A new insight of mechanisms, diagnosis and treatment of diabetic cardiomyopathy. Endocrine 41(3):398–409
Zhang Y, Huang ZJ, Dai DZ, Feng Y, Na T, Tang XY, Dai Y (2008) Downregulated FKBP12.6 expression and upregulated endothelin signaling contribute to elevated diastolic calcium and arrhythmogenesis in rat cardiomyopathy produced by l-thyroxin. Int J Cardiol 130(3):463–471
Zhang Y, Liu W, Ma C, Geng J, Li Y, Li S, Yu F, Zhang X, Cong B (2012) Endoplasmic reticulum stress contributes to CRH-induced hippocampal neuron apoptosis. Exp Cell Res 318(6):732–740
Zheng YF, Dai DZ, Dai Y (2010) NaHS ameliorates diabetic vascular injury by correcting depressed connexin 43 and 40 in the vasculature in streptozotocin-injected rats. J Pharm Pharmacol 62(5):615–621
Ziolo MT, Kohr MJ, Wang H (2008) Nitric oxide signaling and the regulation of myocardial function. J Mol Cell Cardiol 45(5):625–632
Acknowledgments
This work was supported by National Natural Science Foundation of China, no. 81070145.
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
F.H. Shi and Y.S. Cheng contributed to the paper equally as co-first authors.
Rights and permissions
About this article
Cite this article
Shi, F.H., Cheng, Y.S., Dai, D.Z. et al. Depressed calcium-handling proteins due to endoplasmic reticulum stress and apoptosis in the diabetic heart are attenuated by argirein. Naunyn-Schmiedeberg's Arch Pharmacol 386, 521–531 (2013). https://doi.org/10.1007/s00210-013-0852-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-013-0852-5