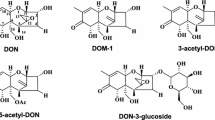
Abstract
Mycotoxins are the most frequently occurring natural contaminants in human and animal diet. Among them, deoxynivalenol (DON), produced by Fusarium, is one of the most prevalent and thus represents an important health risk. Recent detection methods revealed new mycotoxins and new molecules derivated from the “native” mycotoxins. The main derivates of DON are the acetylated forms produced by the fungi (3- and 15-acetyl-DON), the biologically “modified” forms produced by the plant (deoxynivalenol-3-β-d-glucopyranoside), or after bacteria transformation (de-epoxy DON, 3-epi-DON and 3-keto-DON) as well as the chemically “modified” forms (norDON A-C and DON-sulfonates). High proportions of acetylated and modified forms of DON co-occur with DON, increasing the exposure and the health risk. DON and its acetylated and modified forms are rapidly absorbed following ingestion. At the molecular level, DON binds to the ribosome, induces a ribotoxic stress leading to the activation of MAP kinases, cellular cell-cycle arrest and apoptosis. The toxic effects of DON include emesis and anorexia, alteration of intestinal and immune functions, reduced absorption of the nutrients as well as increased susceptibility to infection and chronic diseases. In contrast to DON, very little information exists concerning the acetylated and modified forms; some can be converted back to DON, their ability to bind to the ribosome and to induce cellular effects varies according to the toxin. Except for the acetylated forms, their toxicity and impact on human and animal health are poorly documented.





Similar content being viewed by others
Abbreviations
- Acetyl-DON:
-
Acetyl-deoxynivalenol
- 3-acetyl-DON:
-
3-Acetyl deoxynivalenol
- 15-acetyl-DON:
-
15-Acetyl deoxynivalenol
- CAT:
-
Catalase
- CDK:
-
Cyclin-dependent kinase
- D3G:
-
Deoxynivalenol-3-β-d-glucopyranoside
- DOM-1:
-
De-epoxy DON
- DON:
-
Deoxynivalenol
- DONS:
-
DON sulfate
- ERK1/2:
-
Extracellular signal-regulated kinase 1and 2
- Hck:
-
Hematopoietic cell kinase (Tyrosine protein kinase)
- IEC:
-
Intestinal epithelial cells
- IPEC:
-
Intestinal porcine epithelial cells
- GLUT5:
-
d-Fructose Transporter Glucose Transporter-5
- Ig:
-
Immunoglobulin
- iNOS:
-
Nitric oxide synthase
- JNK:
-
c-Jun N-terminal kinase
- MAP Kinase:
-
Mitogen-activated protein kinase
- NF-κB:
-
Nuclear factor-κB
- NO:
-
Nitric oxide
- PKR:
-
Protein kinase R
- ROS:
-
Reactive oxygen species
- RNS:
-
Reactive nitrogen species
- SGLT1:
-
d-Glucose/d-galactose sodium-dependent transporter
- SOD:
-
Superoxidase dismutase
- TEER:
-
Trans-epithelial electrical resistance
References
Abbas HK, Mirocha CJ, Tuite J (1986) Natural occurrence of deoxynivalenol, 15-acetyl-deoxynivalenol, and zearalenone in refusal factor corn stored since 1972. Appl Environ Microbiol 51:841–843
Ajandouz EH, Berdah S, Moutardier V et al (2016) Hydrolytic Fate of 3/15-acetyldeoxynivalenol in Humans: specific Deacetylation by the Small Intestine and Liver Revealed Using in Vitro and ex Vivo Approaches. Toxins. doi:10.3390/toxins8080232
Akbari P, Braber S, Gremmels H et al (2014) Deoxynivalenol: a trigger for intestinal integrity breakdown. FASEB J 28:2414–2429. doi:10.1096/fj.13-238717
Akbari P, Braber S, Varasteh S et al (2016) The intestinal barrier as an emerging target in the toxicological assessment of mycotoxins. Arch Toxicol. doi:10.1007/s00204-016-1794-8
Alassane-Kpembi I, Kolf-Clauw M, Gauthier T et al (2013) New insights into mycotoxin mixtures: the toxicity of low doses of Type B trichothecenes on intestinal epithelial cells is synergistic. Toxicol Appl Pharmacol 272:191–198. doi:10.1016/j.taap.2013.05.023
Alassane-Kpembi I, Puel O, Oswald IP (2015) Toxicological interactions between the mycotoxins deoxynivalenol, nivalenol and their acetylated derivatives in intestinal epithelial cells. Arch Toxicol 89:1337–1346. doi:10.1007/s00204-014-1309-4
Alassane-Kpembi I, Schatzmayr G, Taranu I et al (2016) Mycotoxins co-contamination: methodological aspects and biological relevance of combined toxicity studies. Crit Rev Food Sci Nutr. doi:10.1080/10408398.2016.1140632
Amuzie CJ, Shinozuka J, Pestka JJ (2009) Induction of suppressors of cytokine signaling by the trichothecene deoxynivalenol in the mouse. Toxicol Sci 111:277–287. doi:10.1093/toxsci/kfp150
Antonissen G, Van Immerseel F, Pasmans F et al (2014) The mycotoxin deoxynivalenol predisposes for the development of Clostridium perfringens-induced necrotic enteritis in broiler chickens. PLoS One 9:e108775. doi:10.1371/journal.pone.0108775
Antonissen G, Van Immerseel F, Pasmans F et al (2015) Mycotoxins deoxynivalenol and fumonisins alter the extrinsic component of intestinal barrier in broiler chickens. J Agric Food Chem 63:10846–10855. doi:10.1021/acs.jafc.5b04119
Awad WA, Zentek J (2015) The feed contaminant deoxynivalenol affects the intestinal barrier permeability through inhibition of protein synthesis. Arch Toxicol. 89:961-965. doi:10.1007/s00204-014-1284-9
Awad WA, Razzazi-Fazeli E, Bohm J, Zentek J (2008) Effects of B-trichothecenes on luminal glucose transport across the isolated jejunal epithelium of broiler chickens. J Anim Physiol Anim Nutr 92:225–230. doi:10.1111/j.1439-0396.2007.00709.x
Awad WA, Ghareeb K, Dadak A et al (2012) Genotoxic effects of deoxynivalenol in broiler chickens fed low-protein feeds. Poult Sci 91:550–555. doi:10.3382/ps.2011-01742
Awad WA, Ghareeb K, Zentek J (2014) Mechanisms underlying the inhibitory effect of the feed contaminant deoxynivalenol on glucose absorption in broiler chickens. Vet J 202:188–190. doi:10.1016/j.tvjl.2014.06.012
Azcona-Olivera JI, Ouyang Y, Murtha J et al (1995) Induction of cytokine mRNAs in mice after oral exposure to the trichothecene vomitoxin (deoxynivalenol): relationship to toxin distribution and protein synthesis inhibition. Toxicol Appl Pharmacol 133:109–120. doi:10.1006/taap.1995.1132
Behrens M, Huwel S, Galla H-J, Humpf H-U (2015) Blood-brain barrier effects of the fusarium mycotoxins deoxynivalenol, 3 acetyldeoxynivalenol, and moniliformin and their transfer to the brain. PLoS One 10:e0143640. doi:10.1371/journal.pone.0143640
Bensassi F, El Golli-Bennour E, Abid-Essefi S et al (2009) Pathway of deoxynivalenol-induced apoptosis in human colon carcinoma cells. Toxicology 264:104–109. doi:10.1016/j.tox.2009.07.020
Berthiller F, Dall’asta C, Corradini R et al (2009) Occurrence of deoxynivalenol and its 3-beta-d-glucoside in wheat and maize. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 26:507–511. doi:10.1080/02652030802555668
Berthiller F, Crews C, Dall’asta C et al (2013) Masked mycotoxins: a review. Mol Nutr Food Res 57:165–186. doi:10.1002/mnfr.201100764
Beyer M, Danicke S, Rohweder D, Humpf H-U (2010) Determination of deoxynivalenol-sulfonate (DONS) in cereals by hydrophilic interaction chromatography coupled to tandem mass spectrometry. Mycotoxin Res 26:109–117. doi:10.1007/s12550-010-0046-7
Bianco G, Fontanella B, Severino L et al (2012) Nivalenol and deoxynivalenol affect rat intestinal epithelial cells: a concentration related study. PLoS One 7:e52051. doi:10.1371/journal.pone.0052051
Bol-Schoenmakers M, Braber S, Akbari P et al (2016) The mycotoxin deoxynivalenol facilitates allergic sensitization to whey in mice. Mucosal Immunol. doi:10.1038/mi.2016.13
Bondy GS, Pestka JJ (1991) Dietary exposure to the trichothecene vomitoxin (deoxynivalenol) stimulates terminal differentiation of Peyer’s patch B cells to IgA secreting plasma cells. Toxicol Appl Pharmacol 108:520–530. doi:10.1016/0041-008x(91)90098-y
Bony S, Carcelen M, Olivier L, Devaux A (2006) Genotoxicity assessment of deoxynivalenol in the Caco-2 cell line model using the Comet assay. Toxicol Lett 166:67–76. doi:10.1016/j.toxlet.2006.04.010
Bracarense APFL, Lucioli J, Grenier B et al (2012) Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br J Nutr 107:1776–1786. doi:10.1017/S0007114511004946
Bracarense APFL, Basso KM, Silva EO, Oswald IP (2016) Deoxynivalenol in liver and lymphoid organs of rats effects of dose and duration on immunohistological changes. World Mycotoxins J. doi:10.3920/wmj2016.2094
Bretz M, Beyer M, Cramer B et al (2006) Thermal degradation of the Fusarium mycotoxin deoxynivalenol. J Agric Food Chem 54:6445–6451. doi:10.1021/jf061008g
Broekaert N, Devreese M, De Baere S et al (2015a) Modified Fusarium mycotoxins unmasked: from occurrence in cereals to animal and human excretion. Food Chem Toxicol 80:17–31. doi:10.1016/j.fct.2015.02.015
Broekaert N, Devreese M, De Mil T et al (2015b) Oral bioavailability, hydrolysis, and comparative toxicokinetics of 3-acetyldeoxynivalenol and 15-acetyldeoxynivalenol in broiler chickens and pigs. J Agric Food Chem 63:8734–8742. doi:10.1021/acs.jafc.5b03270
Broekaert N, Devreese M, van Bergen T et al (2016) In vivo contribution of deoxynivalenol-3-beta-d-glucoside to deoxynivalenol exposure in broiler chickens and pigs: oral bioavailability, hydrolysis and toxicokinetics. Arch Toxicol. doi:10.1007/s00204-016-1710-2
Canady RA, Coker RD, Rgan SK et al (2001) Deoxynivalenol. Safety evaluation of certain mycotoxins in food. WHO Food Addit Ser 47:420–555
Cano PM, Seeboth J, Meurens F et al (2013) Deoxynivalenol as a new factor in the persistence of intestinal inflammatory diseases: an emerging hypothesis through possible modulation of Th17-mediated response. PLoS One 8:e53647. doi:10.1371/journal.pone.0053647
Cheat S, Gerez JR, Cognie J et al (2015) Nivalenol has a greater impact than deoxynivalenol on pig jejunum mucosa in vitro on explants and in vivo on intestinal loops. Toxins 7:1945–1961. doi:10.3390/toxins7061945
Chung YJ, Zhou HR, Pestka JJ (2003) Transcriptional and posttranscriptional roles for p38 mitogen-activated protein kinase in upregulation of TNF-alpha expression by deoxynivalenol (vomitoxin). Toxicol Appl Pharmacol 193:188–201. doi:10.1016/S0041-008X(03)00299-0
Cobb MH (1999) MAP kinase pathways. Prog Biophys Mol Biol 71:479–500. doi:10.1016/S0079-6107(98)00056-X
Collins TFX, Sprando RL, Black TN et al (2006) Effects of deoxynivalenol (DON, vomitoxin) on in utero development in rats. Food Chem Toxicol 44:747–757. doi:10.1016/j.fct.2005.10.007
European Commission (2007) Commission Regulation (EC) No 1126/2007 of 28 September 2007 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Off J Eur Union L 255(2007):14–17
Cote LM, Dahlem AM, Yoshizawa T et al (1986) Excretion of deoxynivalenol and its metabolite in milk, urine, and feces of lactating dairy cows. J Dairy Sci 69:2416–2423. doi:10.3168/jds.S0022-0302(86)80681-6
Cote LM, Buck W, Jeffery E (1987) Lack of hepatic microsomal metabolism of deoxynivalenol and its metabolite, DOM-1. Food Chem Toxicol 25:291–295. doi:10.1016/0278-6915(87)90125-6
Dall’Erta A, Cirlini M, Dall’Asta M et al (2013) Masked mycotoxins are efficiently hydrolyzed by human colonic microbiota releasing their aglycones. Chem Res Toxicol 26:305–312. doi:10.1021/tx300438c
Danicke S, Brezina U (2013) Kinetics and metabolism of the Fusarium toxin deoxynivalenol in farm animals: consequences for diagnosis of exposure and intoxication and carry over. Food Chem Toxicol 60:58–75. doi:10.1016/j.fct.2013.07.017
Danicke S, Valenta H, Doll S (2004) On the toxicokinetics and the metabolism of deoxynivalenol (DON) in the pig. Arch Anim Nutr 58:169–180. doi:10.1080/00039420410001667548
Danicke S, Brussow K-P, Goyarts T et al (2007) On the transfer of the Fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) from the sow to the full-term piglet during the last third of gestation. Food Chem Toxicol 45:1565–1574. doi:10.1016/j.fct.2007.02.016
Danicke S, Hegewald AK, Kahlert S et al (2010) Studies on the toxicity of deoxynivalenol (DON), sodium metabisulfite, DON-sulfonate (DONS) and de-epoxy-DON for porcine peripheral blood mononuclear cells and the Intestinal Porcine Epithelial Cell lines IPEC-1 and IPEC-J2, and on effects of DON and DONS. Food Chem Toxicol 48:2154–2162. doi:10.1016/j.fct.2010.05.022
De Angelis E, Monaci L, Visconti A (2014) Investigation on the stability of deoxynivalenol and DON-3 glucoside during gastro-duodenal in vitro digestion of a naturally contaminated bread model food. Food Control 43:270–275. doi:10.1016/j.foodcont.2014.03.032
De Nijs M, Van den Top HJ, Portier L et al (2012) Digestibility and absorption of deoxynivalenol-3-ß-glucoside in in vitro models. World Mycotoxin J 5:319–324. doi:10.3920/WMJ2012.1430
Del Regno M, Adesso S, Popolo A et al (2015) Nivalenol induces oxidative stress and increases deoxynivalenol pro-oxidant effect in intestinal epithelial cells. Toxicol Appl Pharmacol 285:118–127. doi:10.1016/j.taap.2015.04.002
Diesing AK, Nossol C, Danicke S et al (2011) Vulnerability of polarised intestinal porcine epithelial cells to mycotoxin deoxynivalenol depends on the route of application. PLoS One 6:e17472. doi:10.1371/journal.pone.0017472
Dinu D, Bodea GO, Ceapa CD et al (2011) Adapted response of the antioxidant defense system to oxidative stress induced by deoxynivalenol in Hek-293 cells. Toxicon 57:1023–1032. doi:10.1016/j.toxicon.2011.04.006
EFSA (2014) Scientific opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA J 12:3916. doi:10.2903/j.efsa.2014.3916
Eriksen GS, Alexander J (1998) Fusarium toxins in cereals—a risk assessment. Nordic Council of Ministers, Copenhagen, Denmark.
Eriksen GS, Pettersson H (2004) Toxicological evaluation of trichothecenes in animal feed. Anim Feed Sci Technol 114:205–239. doi:10.1016/j.anifeedsci.2003.08.008
Eriksen GS, Pettersson H, Johnsen K, Lindberg JE (2002) Transformation of trichothecenes in ileal digesta and faeces from pigs. Arch Tierernahr 56:263–274. doi:10.1080/00039420214343
Eriksen GS, Pettersson H, Lindberg JE (2003) Absorption, metabolism and excretion of 3-acetyl DON in pigs. Arch Tierernahr 57:335–345. doi:10.1080/00039420310001607699
Eriksen GS, Pettersson H, Lundh T (2004) Comparative cytotoxicity of deoxynivalenol, nivalenol, their acetylated derivatives and de-epoxy metabolites. Food Chem Toxicol 42:619–624. doi:10.1016/j.fct.2003.11.006
FDA (2010) Guidance for industry and FDAi advisory levels for deoxynivalenol (DON) in finished wheat products for human consumption and grains and grain by-products used for animal feed. http://www.fda.gov/downloads/Food/GuidanceRegulation/UCM217558.pdf
Fioramonti J, Dupuy C, Dupuy J, Bueno L (1993) The mycotoxin, deoxynivalenol, delays gastric emptying through serotonin-3 receptors in rodents. J Pharmacol Exp Ther 266:1255–1260
Flannery BM, Clark ES, Pestka JJ (2012) Anorexia induction by the trichothecene deoxynivalenol (vomitoxin) is mediated by the release of the gut satiety hormone peptide YY. Toxicol Sci 130:289–297. doi:10.1093/toxsci/kfs255
Forsell JH, Pestka JJ (1985) Relation of 8-ketotrichothecene and zearalenone analog structure to inhibition of mitogen-induced human lymphocyte blastogenesis. Appl Environ Microbiol 50:1304–1307
Forsell JH, Jensen R, Tai JH et al (1987) Comparison of acute toxicities of deoxynivalenol (vomitoxin) and 15-acetyldeoxynivalenol in the B6C3F1 mouse. Food Chem Toxicol 25:155–162. doi:10.1016/0278-6915(87)90149-9
Frankic T, Pajk T, Rezar V et al (2006) The role of dietary nucleotides in reduction of DNA damage induced by T-2 toxin and deoxynivalenol in chicken leukocytes. Food Chem Toxicol 44:1838–1844. doi:10.1016/j.fct.2006.06.002
Fuchs E, Binder EM, Heidler D, Krska R (2002) Structural characterization of metabolites after the microbial degradation of type A trichothecenes by the bacterial strain BBSH 797. Food Addit Contam 19:379–386. doi:10.1080/02652030110091154
Gaige S, Bonnet MS, Tardivel C et al (2013) c-Fos immunoreactivity in the pig brain following deoxynivalenol intoxication: focus on NUCB2/nesfatin-1 expressing neurons. Neurotoxicology 34:135–149. doi:10.1016/j.neuro.2012.10.020
Gareis M, Bauer J, Thiem J et al (1990) Cleavage of zearalenone-glycoside, a “masked” mycotoxin, during digestion in swine. Zentralbl Veterinarmed B 37:236–240. doi:10.1111/j.1439-0450.1990.tb01052.x
Garreau de Loubresse N, Prokhorova I, Holtkamp W et al (2014) Structural basis for the inhibition of the eukaryotic ribosome. Nature 513:517–522. doi:10.1038/nature13737
Ghareeb K, Awad WA, Böhm J, Zebeli Q (2015) Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: poultry and swine. J Appl Toxicol 35:327–337. doi:10.1002/jat.3083
Ghareeb K, Awad WA, Zebeli Q, Bohm J (2016) Deoxynivalenol in chicken feed alters the vaccinal immune response and clinical biochemical serum parameters but not the intestinal and carcass characteristics. J Anim Physiol Anim Nutr 100:53–60. doi:10.1111/jpn.12328
Gilbert J, Brûlé-Babel A, Guerrieri AT et al (2014) Ratio of 3-ADON and 15-ADON isolates of Fusarium graminearum recovered from wheat kernels in Manitoba from 2008 to 2012. Can J Plant Pathol 36:54–63. doi:10.1080/07060661.2014.887033
Girardet C, Bonnet MS, Jdir R et al (2011a) The food-contaminant deoxynivalenol modifies eating by targeting anorexigenic neurocircuitry. PLoS One 6:e26134. doi:10.1371/journal.pone.0026134
Girardet C, Bonnet MS, Jdir R et al (2011b) Central inflammation and sickness-like behavior induced by the food contaminant deoxynivalenol: a PGE2-independent mechanism. Toxicol Sci 124:179–191. doi:10.1093/toxsci/kfr219
Girgis GN, Sharif S, Barta JR et al (2008) Immunomodulatory effects of feed-borne Fusarium mycotoxins in chickens infected with coccidia. Exp Biol Med 233:1411–1420. doi:10.3181/0805-RM-173
Girgis GN, Barta JR, Brash M, Smith TK (2010) Morphologic changes in the intestine of broiler breeder pullets fed diets naturally contaminated with Fusarium mycotoxins with or without coccidial challenge. Avian Dis 54:67–73. doi:10.1637/8945-052809-Reg.1
Girish CK, Smith TK, Boermans HJ, Karrow NA (2008) Effects of feeding blends of grains naturally contaminated with Fusarium mycotoxins on performance, hematology, metabolism, and immunocompetence of turkeys. Poult Sci 87:421–432. doi:10.3382/ps.2007-00181
Gouze ME, Laffitte J, Rouimi P et al (2006) Effect of various doses of deoxynivalenol on liver xenobiotic metabolizing enzymes in mice. Food Chem Toxicol 44:476–483. doi:10.1016/j.fct.2005.08.020
Goyarts T, Danicke S (2006) Bioavailability of the Fusarium toxin deoxynivalenol (DON) from naturally contaminated wheat for the pig. Toxicol Lett 163:171–182. doi:10.1016/j.toxlet.2005.10.007
Goyarts T, Danicke S, Brussow K-P et al (2007) On the transfer of the Fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) from sows to their fetuses during days 35-70 of gestation. Toxicol Lett 171:38–49. doi:10.1016/j.toxlet.2007.04.003
Gratz SW, Duncan G, Richardson AJ (2013) The human fecal microbiota metabolizes deoxynivalenol and deoxynivalenol-3-glucoside and may be responsible for urinary de-epoxy-deoxynivalenol. Appl Environ Microbiol 79:1821–1825. doi:10.1128/AEM.02987-12
Graziani F, Pujol A, Nicoletti C et al (2015) The food-associated ribotoxin deoxynivalenol modulates inducible NO synthase in human intestinal cell model. Toxicol Sci 145:372–382. doi:10.1093/toxsci/kfv058
Grenier B, Loureiro-Bracarense AP, Lucioli J et al (2011) Individual and combined effects of subclinical doses of deoxynivalenol and fumonisins in piglets. Mol Nutr Food Res 55:761–771. doi:10.1002/mnfr.201000402
Guan S, He J, Young JC, Zhu H, Li XZ, Ji C, Zhou T (2009) Transformation of trichothecene mycotoxins by microorganisms from fish digesta. Aquaculture 290:290–295. doi:10.1016/j.aquaculture.2009.02.037
Hara-Kudo Y, Sugita-Konoshi Y, Kasuga F, Kumagai S (1996) Effects of deoxynivalenol on Salmonella enteritidis infection. Mycotoxins 42:51–55. doi:10.2520/myco1975.1996.51
He P, Young LG, Forsberg C (1992) Microbial transformation of deoxynivalenol (vomitoxin). Appl Environ Microbiol 58:3857–3863
He P, Young LG, Forsberg C (1993) Microbially detoxified vomitoxin-contaminated corn for young pigs. J Anim Sci 71:963–967. doi:10.2527/1993.714963x
He J, Zhou T, Young JC et al (2010) Chemical and biological transformations for detoxification of trichothecene mycotoxins in human and animal food chains: a review. Trends Food Sci Technol 21:67–76. doi:10.1016/j.tifs.2009.08.002
He K, Zhou HR, Pestka JJ (2012) Targets and intracellular signaling mechanisms for deoxynivalenol-induced ribosomal RNA cleavage. Toxicol Sci 127:382-390. doi:10.1093/toxsci/kfs134
He JW, Bondy GS, Zhou T et al (2015) Toxicology of 3-epi-deoxynivalenol, a deoxynivalenol-transformation product by Devosia mutans 17-2-E-8. Food Chem Toxicol 84:250–259. doi:10.1016/j.fct.2015.09.003
Hedman R, Pettersson H (1997) Transformation of nivalenol by gastrointestinal microbes. Arch Tierernahr 50:321–329. doi:10.1080/17450399709386142
Humpf H-U, Voss KA (2004) Effects of thermal food processing on the chemical structure and toxicity of fumonisin mycotoxins. Mol Nutr Food Res 48:255–269. doi:10.1002/mnfr.200400033
Hymery N, Sibiril Y, Parent-Massin D (2006) In vitro effects of trichothecenes on human dendritic cells. Toxicol In Vitro 20:899–909. doi:10.1016/j.tiv.2006.01.015
IARC (1993) Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. In: Monographs on the evaluation of carcinogenic risks to humans, Vol 56. International Agency for Research on Cancer, World Health Organization, Lyon, France, pp 397–444
Ikunaga Y, Sato I, Grond S et al (2011) Nocardioides sp. strain WSN05-2, isolated from a wheat field, degrades deoxynivalenol, producing the novel intermediate 3-epi-deoxynivalenol. Appl Microbiol Biotechnol 89:419–427. doi:10.1007/s00253-010-2857-z
Islam R, Zhou T, Young JC et al (2012) Aerobic and anaerobic de-epoxydation of mycotoxin deoxynivalenol by bacteria originating from agricultural soil. World J Microbiol Biotechnol 28:7–13. doi:10.1007/s11274-011-0785-4
Ito M, Sato I, Ishizaka M et al (2013) Bacterial cytochrome P450 system catabolizing the Fusarium toxin deoxynivalenol. Appl Environ Microbiol 79:1619–1628. doi:10.1128/AEM.03227-12
Kadota T, Furusawa H, Hirano S et al (2013) Comparative study of deoxynivalenol, 3-acetyldeoxynivalenol, and 15-acetyldeoxynivalenol on intestinal transport and IL-8 secretion in the human cell line Caco-2. Toxicol Vitro 27:1888–1895. doi:10.1016/j.tiv.2013.06.003
Kalaiselvi P, Rajashree K, Priya LB, Padma VV (2013) Cytoprotective effect of epigallocatechin-3-gallate against deoxynivalenol-induced toxicity through anti-oxidative and anti-inflammatory mechanisms in HT-29 cells. Food Chem Toxicol 56:110–118. doi:10.1016/j.fct.2013.01.042
Karlovsky P (2011) Biological detoxification of the mycotoxin deoxynivalenol and its use in genetically engineered crops and feed additives. Appl Microbiol Biotechnol 91:491–504. doi:10.1007/s00253-011-3401-5
Kasali OB, Schiefer HB, Hancock DS et al (1985) Subacute toxicity of dietary 3-acetyldeoxynivalenol in mice. Can J Comp Med 49:319–322
Keese C, Meyer U, Valenta H et al (2008) No carry over of unmetabolised deoxynivalenol in milk of dairy cows fed high concentrate proportions. Mol Nutr Food Res 52:1514–1529. doi:10.1002/mnfr.200800077
Khera KS, Arnold DL, Whalen C et al (1984) Vomitoxin (4-deoxynivalenol): effects on reproduction of mice and rats. Toxicol Appl Pharmacol 74:345–356. doi:10.1016/0041-008X(84)90288-6
Khera KS, Whalen C, Angers G (1986) A teratology study on vomitoxin (4-deoxynivalenol) in rabbits. Food Chem Toxicol 24:421–424. doi:10.1016/0278-6915(86)90207-3
Kimura M, Kaneko I, Komiyama M et al (1998) Trichothecene 3-O-acetyltransferase protects both the producing organism and transformed yeast from related mycotoxins. Cloning and characterization of Tri101. J Biol Chem 273:1654-166. doi:10.1074/jbc.273.3.1654
King RR, Mcqueen RE, Levesque D, Greenhalgh R (1984) Transformation of deoxynivalenol (vomitoxin) by rumen microorganisms. J Agri Food Chem 32:1181–1183. doi: 10.1021/jf00125a061
Kluger B, Bueschl C, Lemmens M et al (2013) Stable isotopic labelling-assisted untargeted metabolic profiling reveals novel conjugates of the mycotoxin deoxynivalenol in wheat. Anal Bioanal Chem 405:5031–5036. doi:10.1007/s00216-012-6483-8
Kolf-Clauw M, Castellote J, Joly B et al (2009) Development of a pig jejunal explant culture for studying the gastrointestinal toxicity of the mycotoxin deoxynivalenol: histopathological analysis. Toxicol In Vitro 23:1580–1584. doi:10.1016/j.tiv.2009.07.015
Kollarczik B, Gareis M, Hanelt M (1994) In vitro transformation of the Fusarium mycotoxins deoxynivalenol and zearalenone by the normal gut microflora of pigs. Nat Toxins 2:105–110. doi:10.1002/nt.2620020303
Krishnaswamy R, Devaraj SN, Padma VV (2010) Lutein protects HT-29 cells against Deoxynivalenol-induced oxidative stress and apoptosis: prevention of NF-kappaB nuclear localization and down regulation of NF-kappaB and Cyclo-Oxygenase-2 expression. Free Radic Biol Med 49:50–60. doi:10.1016/j.freeradbiomed.2010.03.016
Lake BG, Phillips JC, Walters DG et al (1987) Studies on the metabolism of deoxynivalenol in the rat. Food Chem Toxicol 25:589–592. doi:10.1016/0278-6915(87)90019-6
Larsen JC, Hunt J, Perrin I, Ruckenbauer P (2004) Workshop on trichothecenes with a focus on DON: summary report. Toxicol Lett 153:1–22. doi:10.1016/j.toxlet.2004.04.020
Lattanzio VMT, Solfrizzo M, De Girolamo A et al (2011) LC-MS/MS characterization of the urinary excretion profile of the mycotoxin deoxynivalenol in human and rat. J Chromatogr B Anal Technol Biomed Life Sci 879:707–715. doi:10.1016/j.jchromb.2011.01.029
Li M, Cuff CF, Pestka JJ (2005) Modulation of murine host response to enteric reovirus infection by the trichothecene deoxynivalenol. Toxicol Sci 87:134–145. doi:10.1093/toxsci/kfi225
Li M, Harkema JR, Cuff CF, Pestka JJ (2007) Deoxynivalenol exacerbates viral bronchopneumonia induced by respiratory reovirus infection. Toxicol Sci 95:412–426. doi:10.1093/toxsci/kfl153
Li D, Ma H, Ye Y et al (2014a) Deoxynivalenol induces apoptosis in mouse thymic epithelial cells through mitochondria-mediated pathway. Environ Toxicol Pharmacol 38:163–171. doi:10.1016/j.etap.2014.05.015
Li D, Ye Y, Lin S et al (2014b) Evaluation of deoxynivalenol-induced toxic effects on DF-1 cells in vitro: cell-cycle arrest, oxidative stress, and apoptosis. Environ Toxicol Pharmacol 37:141–149. doi:10.1016/j.etap.2013.11.015
Lucioli J, Pinton P, Callu P et al (2013) The food contaminant deoxynivalenol activates the mitogen activated protein kinases in the intestine: interest of ex vivo models as an alternative to in vivo experiments. Toxicon 66:31–36. doi:10.1016/j.toxicon.2013.01.024
Ma YY, Guo HW (2008) Mini-review of studies on the carcinogenicity of deoxynivalenol. Environ Toxicol Pharmacol 25:1–9. doi:10.1016/j.etap.2007.09.007
Ma Y, Zhang A, Shi Z et al (2012) A mitochondria-mediated apoptotic pathway induced by deoxynivalenol in human colon cancer cells. Toxicol Vitr 26:414–420. doi:10.1016/j.tiv.2012.01.010
Malekinejad H, Schoevers EJ, Daemen IJJM et al (2007) Exposure of oocytes to the Fusarium toxins zearalenone and deoxynivalenol causes aneuploidy and abnormal embryo development in pigs. Biol Reprod 77:840–847. doi:10.1095/biolreprod.107.062711
Maresca M (2013) From the gut to the brain: journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins 5:784–820. doi:10.3390/toxins5040784
Maresca M, Fantini J (2010) Some food-associated mycotoxins as potential risk factors in humans predisposed to chronic intestinal inflammatory diseases. Toxicon 56:282–294. doi:10.1016/j.toxicon.2010.04.016
Maresca M, Mahfoud R, Garmy N, Fantini J (2002) The mycotoxin deoxynivalenol affects nutrient absorption in human intestinal epithelial cells. J Nutr 132:2723–2731
Maresca M, Yahi N, Younes-Sakr L et al (2008) Both direct and indirect effects account for the pro-inflammatory activity of enteropathogenic mycotoxins on the human intestinal epithelium: stimulation of interleukin-8 secretion, potentiation of interleukin-1beta effect and increase in the trans-epithelial passage of commensal bacteria. Toxicol Appl Pharmacol 228:84–92. doi:10.1016/j.taap.2007.11.013
Maul R, Warth B, Kant J-S et al (2012) Investigation of the hepatic glucuronidation pattern of the Fusarium mycotoxin deoxynivalenol in various species. Chem Res Toxicol 25:2715–2717. doi:10.1021/tx300348x
Meinild A, Klaerke DA, Loo DD et al (1998) The human Na+-glucose cotransporter is a molecular water pump. J Physiol. doi:10.1111/j.1469-7793.1998.015br.x
Mishra S, Dwivedi PD, Pandey HP, Das M (2014) Role of oxidative stress in Deoxynivalenol induced toxicity. Food Chem Toxicol. 72:20-29. doi:10.1016/j.fct.2014.06.027
Moon Y, Pestka JJ (2002) Vomitoxin-induced cyclooxygenase-2 gene expression in macrophages mediated by activation of ERK and p38 but not JNK mitogen-activated protein kinases. Toxicol Sci 69:373–382. doi:10.1093/toxsci/69.2.373
Morrissey RE, Vesonder RF (1985) Effect of deoxynivalenol (vomitoxin) on fertility, pregnancy, and postnatal development of Sprague-Dawley rats. Appl Environ Microbiol 49:1062–1066
Nagl V, Schwartz H, Krska R et al (2012) Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in rats. Toxicol Lett 213:367–373. doi:10.1016/j.toxlet.2012.07.024
Nagl V, Woechtl B, Schwartz-Zimmermann HE et al (2014) Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in pigs. Toxicol Lett 229:190–197. doi:10.1016/j.toxlet.2014.06.032
Osselaere A, Devreese M, Goossens J et al (2013a) Toxicokinetic study and absolute oral bioavailability of deoxynivalenol, T-2 toxin and zearalenone in broiler chickens. Food Chem Toxicol 51:350–355. doi:10.1016/j.fct.2012.10.006
Osselaere A, Santos R, Hautekiet V et al (2013b) Deoxynivalenol impairs hepatic and intestinal gene expression of selected oxidative stress, tight junction and inflammation proteins in broiler chickens, but addition of an adsorbing agent shifts the effects to the distal parts of the small intestine. PLoS One 8:e69014. doi:10.1371/journal.pone.0069014PONE-D-13-06016
Ossenkopp KP, Hirst M, Rapley WA (1994) Deoxynivalenol (vomitoxin)-induced conditioned taste aversions in rats are mediated by the chemosensitive area postrema. Pharmacol Biochem Behav 47:363–367. doi:10.1016/0091-3057(94)90024-8
Parent-Massin D (2004) Haematotoxicity of trichothecenes. Toxicol Lett 153:75–81. doi:10.1016/j.toxlet.2004.04.024
Paulick M, Rempe I, Kersten S et al (2015a) Effects of increasing concentrations of sodium sulfite on deoxynivalenol and deoxynivalenol sulfonate concentrations of maize kernels and maize meal preserved at various moisture content. Toxins 7:791–811. doi:10.3390/toxins7030791
Paulick M, Winkler J, Kersten S et al (2015b) Studies on the bioavailability of deoxynivalenol (DON) and DON sulfonate (DONS) 1, 2, and 3 in pigs fed with sodium sulfite-treated DON-contaminated maize. Toxins 7:4622–4644. doi:10.3390/toxins7114622
Payros D, Oswald E, Oswald IP (2016) Food contaminant exacerbates the genotoxicity of gut microbiota. (submitted)
Pelyhe C, Kovesi B, Zandoki E et al (2016) Effect of 4-week feeding of deoxynivalenol- or T-2-toxin-contaminated diet on lipid peroxidation and glutathione redox system in the hepatopancreas of common carp (Cyprinus carpio L.). Mycotoxin Res 32:77–83. doi:10.1007/s12550-016-0242-1
Pestka JJ (2003) Deoxynivalenol-induced IgA production and IgA nephropathy-aberrant mucosal immune response with systemic repercussions. Toxicol Lett 140–141:287–295. doi:10.1016/S0378-4274(03)00024-9
Pestka JJ (2007) Deoxynivalenol: toxicity, mechanisms and animal health risks. Anim Feed Sci Technol 137:283–298. doi:10.1016/j.anifeedsci.2007.06.006
Pestka JJ (2008) Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit Contam 25:1128–1140. doi:10.1080/02652030802056626
Pestka JJ (2010) Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch Toxicol 84:663–679. doi:10.1007/s00204-010-0579-8
Pestka JJ, Amuzie CJ (2008) Tissue distribution and proinflammatory cytokine gene expression following acute oral exposure to deoxynivalenol: comparison of weanling and adult mice. Food Chem Toxicol 46:2826–2831. doi:10.1016/j.fct.2008.05.016
Pestka JJ, Smolinski AT (2005) Deoxynivalenol: toxicology and potential effects on humans. J Toxicol Environ Health B Crit Rev 8:39–69. doi:10.1080/10937400590889458
Pestka JJ, Lin WS, Miller ER (1987) Emetic activity of the trichothecene 15-acetyldeoxynivalenol in swine. Food Chem Toxicol 25:855–858. doi:10.1016/0278-6915(87)90264-X
Pestka JJ, Zhou HR, Moon Y, Chung YJ (2004) Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: unraveling a paradox. Toxicol Lett 153:61–73. doi:10.1016/j.toxlet.2004.04.023
Pestka JJ, Uzarski RL, Islam Z (2005) Induction of apoptosis and cytokine production in the Jurkat human T cells by deoxynivalenol: role of mitogen-activated protein kinases and comparison to other 8-ketotrichothecenes. Toxicology 206:207–219. doi:10.1016/j.tox.2004.08.020
Pierron A, Alassane-Kpembi I, Oswald IP (2016a) Impact of mycotoxin on immune response and consequences for pig health. Anim Nutr 2:63–68. doi:10.1016/j.aninu.2016.03.001
Pierron A, Mimoun S, Murate LS et al (2016b) Intestinal toxicity of the masked mycotoxin deoxynivalenol-3-beta-d-glucoside. Arch Toxicol 90:2037–2046. doi:10.1007/s00204-015-1592-8
Pierron A, Mimoun S, Murate LS et al (2016c) Microbial biotransformation of DON: molecular basis for reduced toxicity. Sci Rep 6:29105. doi:10.1038/srep29105
Pinton P, Oswald IP (2014) Effect of deoxynivalenol and other Type B trichothecenes on the intestine: a review. Toxins 6:1615–1643. doi:10.3390/toxins6051615
Pinton P, Accensi F, Beauchamp E et al (2008) Ingestion of deoxynivalenol (DON) contaminated feed alters the pig vaccinal immune responses. Toxicol Lett 177:215–222. doi:10.1016/j.toxlet.2008.01.015
Pinton P, Nougayrede JP, Del Rio JC et al (2009) The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol Appl Pharmacol 237:41–48. doi:10.1016/j.taap.2009.03.003
Pinton P, Tsybulskyy D, Lucioli J et al (2012) Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: differential effects on morphology, barrier function, tight junction proteins, and mitogen-activated protein kinases. Toxicol Sci 130:180–190. doi:10.1093/toxsci/kfs239
Pinton P, Graziani F, Pujol A et al (2015) Deoxynivalenol inhibits the expression by goblet cells of intestinal mucins through a PKR and MAP kinase dependent repression of the resistin-like molecule beta. Mol Nutr Food Res 59:1076–1087. doi:10.1002/mnfr.201500005
Piotrowska M, Slizewska K, Nowak A et al (2014) The effect of experimental fusarium mycotoxicosis on microbiota diversity in porcine ascending colon contents. Toxins 6:2064–2081. doi:10.3390/toxins6072064
Poppenberger B, Berthiller F, Lucyshyn D et al (2003) Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J Biol Chem 278:47905–47914. doi:10.1074/jbc.M307552200M307552200
Prelusky DB, Veira DM, Trenholm HL (1985) Plasma pharmacokinetics of the mycotoxin deoxynivalenol following oral and intravenous administration to sheep. J Environ Sci Health B 20:603–624. doi:10.1080/03601238509372499
Prelusky DB, Veira DM, Trenholm HL, Hartin KE (1986) Excretion profiles of the mycotoxin deoxynivalenol, following oral and intravenous administration to sheep. Fundam Appl Toxicol 6:356–363. doi:10.1016/0272-0590(86)90251-4
Prelusky DB, Hartin KE, Trenholm HL, Miller JD (1988) Pharmacokinetic fate of 14C-labeled deoxynivalenol in swine. Fundam Appl Toxicol 10:276–286
Razafimanjato H, Benzaria A, Taieb N et al (2011) The ribotoxin deoxynivalenol affects the viability and functions of glial cells. Glia 59:1672–1683. doi:10.1002/glia.21214
Rizzo AF, Atroshi F, Ahotupa M et al (1994) Protective effect of antioxidants against free radical-mediated lipid peroxidation induced by DON or T-2 toxin. Zentralbl Veterinarmed A 41:81–90. doi:10.1111/j.1439-0442.1994.tb00070.x
Rodriguez-Carrasco Y, Molto JC, Berrada H, Manes J (2014) A survey of trichothecenes, zearalenone and patulin in milled grain-based products using GC-MS/MS. Food Chem 146:212–219. doi:10.1016/j.foodchem.2013.09.053
Rotter BA, Thompson BK, Prelusky DB, Trenholm HL (1991) Evaluation of potential interactions involving trichothecene mycotoxins using the chick embryotoxicity bioassay. Arch Environ Contam Toxicol 21:621–624
Rychlik M, Humpf H-U, Marko D et al (2014) Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin Res 30:197–205. doi:10.1007/s12550-014-0203-5
Saint-Cyr MJ, Perrin-Guyomard A, Houée P et al (2013) Evaluation of an oral subchronic exposure of deoxynivalenol on the composition of human gut microbiota in a model of human microbiota-associated rats. PLoS One 8:e80578. doi:10.1371/journal.pone.0080578
Sarkanj B, Warth B, Uhlig S et al (2013) Urinary analysis reveals high deoxynivalenol exposure in pregnant women from Croatia. Food Chem Toxicol 62:231–237. doi:10.1016/j.fct.2013.08.043
Sato I, Ito M, Ishizaka M et al (2012) Thirteen novel deoxynivalenol-degrading bacteria are classified within two genera with distinct degradation mechanisms. FEMS Microbiol Lett 327:110–117. doi:10.1111/j.1574-6968.2011.02461.x
Savard C, Provost C, Alvarez F et al (2015) Effect of deoxynivalenol (DON) mycotoxin on in vivo and in vitro porcine circovirus type 2 infections. Vet Microbiol 176:257–267. doi:10.1016/j.vetmic.2015.02.004
Schatzmayr G, Zehner F, Taubel M et al (2006) Microbiologicals for deactivating mycotoxins. Mol Nutr Food Res 50:543–551. doi:10.1002/mnfr.200500181
Schwartz HE, Hametner C, Slavik V et al (2013) Characterization of three deoxynivalenol sulfonates formed by reaction of deoxynivalenol with sulfur reagents. J Agric Food Chem 61:8941–8948. doi:10.1021/jf403438b
Schwartz-Zimmermann HE, Hametner C, Nagl V et al (2014a) Deoxynivalenol (DON) sulfonates as major DON metabolites in rats: from identification to biomarker method development, validation and application. Anal Bioanal Chem 406:7911–7924. doi:10.1007/s00216-014-8252-3
Schwartz-Zimmermann HE, Wiesenberger G, Unbekannt C et al (2014b) Reaction of (conjugated) deoxynivalenol with sulphur reagents—novel metabolites, toxicity and application. World Mycotoxin J 7:187–197. doi:10.3920/WMJ2013.1632
Schwartz-Zimmermann HE, Fruhmann P, Danicke S et al (2015) Metabolism of deoxynivalenol and de-epoxy-deoxynivalenol in broiler chickens, pullets, roosters and turkeys. Toxins 7:4706–4729. doi:10.3390/toxins7114706
Seeling K, Danicke S, Valenta H et al (2006) Effects of Fusarium toxin-contaminated wheat and feed intake level on the biotransformation and carry-over of deoxynivalenol in dairy cows. Food Addit Contam 23:1008–1020. doi:10.1080/02652030600723245
Severino L, Luongo D, Bergamo P et al (2006) Mycotoxins nivalenol and deoxynivalenol differentially modulate cytokine mRNA expression in Jurkat T cells. Cytokine 36:75–82. doi:10.1016/j.cyto.2006.11.006
Shima J, Takase S, Takahashi Y et al (1997) Novel detoxification of the trichothecene mycotoxin deoxynivalenol by a soil bacterium isolated by enrichment culture. Appl Environ Microbiol 63:3825–3830
Singh S, Banerjee S, Chattopadhyay P et al (2015) Deoxynivalenol induces cytotoxicity and genotoxicity in animal primary cell culture. Toxicol Mech Methods 6516:1–8. doi:10.3109/15376516.2015.1006743
Sprando RL, Collins TF, Black TN et al (2005) Characterization of the effect of deoxynivalenol on selected male reproductive endpoints. Food Chem Toxicol 43:623–635. doi:10.1016/j.fct.2004.12.017
Streit E, Naehrer K, Rodrigues I, Schatzmayr G (2013) Mycotoxin occurrence in feed and feed raw materials worldwide: long-term analysis with special focus on Europe and Asia. J Sci Food Agric 93:2892–2899. doi:10.1002/jsfa.6225
Sugita-Konishi Y, Pestka JJ (2001) Differential upregulation of TNF-alpha, IL-6, and IL-8 production by deoxynivalenol (vomitoxin) and other 8-ketotrichothecenes in a human macrophage model. J Toxicol Environ Health A 64:619–636. doi:10.1080/152873901753246223
Sugita-Konishi Y, Park BJ, Kobayashi-Hattori K et al (2006) Effect of cooking process on the deoxynivalenol content and its subsequent cytotoxicity in wheat products. Biosci Biotechnol Biochem 70:1764–1768. doi:10.1271/bbb.50571
Sundstol Eriksen G, Pettersson H (2003) Lack of de-epoxidation of type B trichothecenes in incubates with human faeces. Food Addit Contam 20:579–582. doi:10.1080/0265203031000102573
Swanson SP, Nicoletti J, Rood HDJ et al (1987) Metabolism of three trichothecene mycotoxins, T-2 toxin, diacetoxyscirpenol and deoxynivalenol, by bovine rumen microorganisms. J Chromatogr 414:335–342
Taranu I, Marina DE, Burlacu R et al (2010) Comparative aspects of in vitro proliferation of human and porcine lymphocytes exposed to mycotoxins. Arch Anim Nutr 64:383–393. doi:10.1080/1745039X.2010.492140
Tomar RS, Blakley BR, Schiefer HB, DeCoteau WE (1986) In vitro effects of 3-acetyl-deoxynivalenol on the immune response of human peripheral blood lymphocytes. Int J Immunopharmacol 8:125–130. doi:10.1016/0192-0561(86)90051-2
Tomar RS, Blakley BR, DeCoteau WE (1987) Immunological responsiveness of mouse spleen cells after in vivo or in vitro exposure to 3-acetyldeoxynivalenol. Food Chem Toxicol. 25:393–8. doi:10.1016/0278-6915(87)90175-X
Turner PC, Hopton RP, Lecluse Y et al (2010) Determinants of urinary deoxynivalenol and de-epoxy deoxynivalenol in male farmers from Normandy, France. J Agric Food Chem 58:5206–5212. doi:10.1021/jf100892v
Uhlig S, Ivanova L, Faeste CK (2016) Correction to enzyme-assisted synthesis and structural characterization of the 3-, 8-, and 15-glucuronides of deoxynivalenol. J Agric Food Chem 64:3732. doi:10.1021/acs.jafc.6b01413
van der Flier LG, Clevers H (2009) Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71:241–260. doi:10.1146/annurev.physiol.010908.163145
Vandenbroucke V, Croubels S, Verbrugghe E et al (2009) The mycotoxin deoxynivalenol promotes uptake of Salmonella Typhimurium in porcine macrophages, associated with ERK1/2 induced cytoskeleton reorganization. Vet Res 40:64. doi:10.1051/vetres/2009045
Vandenbroucke V, Croubels S, Martel A et al (2011) The mycotoxin deoxynivalenol potentiates intestinal inflammation by salmonella typhimurium in porcine ileal loops. PLoS One 6:e23871. doi:10.1371/journal.pone.0023871
Versilovskis A, Bartkevics V (2012) Stability of sterigmatocystin during the bread making process and its occurrence in bread from the Latvian market. Mycotoxin Res 28:123–129. doi:10.1007/s12550-012-0124-0
Volkl A, Vogler B, Schollenberger M, Karlovsky P (2004) Microbial detoxification of mycotoxin deoxynivalenol. J Basic Microbiol 44:147–156. doi:10.1002/jobm.200310353
Wache YJ, Hbabi-Haddioui L, Guzylack-Piriou L et al (2009a) The mycotoxin Deoxynivalenol inhibits the cell surface expression of activation markers in human macrophages. Toxicology 262:239–244. doi:10.1016/j.tox.2009.06.014
Wache YJ, Valat C, Postollec G et al (2009b) Impact of deoxynivalenol on the intestinal microflora of pigs. Int J Mol Sci 10:1–17. doi:10.3390/ijms10010001
Wan D, Huang L, Pan Y et al (2013) Metabolism, distribution, and excretion of deoxynivalenol with combined techniques of radiotracing, high-performance liquid chromatography ion trap time-of-flight mass spectrometry, and online radiometric detection. J Agric Food Chem 62:288–296. doi:10.1021/jf4047946
Wu X, Kohut M, Cunnick J et al (2009) Deoxynivalenol suppresses circulating and splenic leukocyte subpopulations in BALB/c mice: dose response, time course and sex differences. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 26:1070–1080. doi:10.1080/02652030902832959
Wu W, Flannery BM, Sugita-Konishi Y et al (2012) Comparison of murine anorectic responses to the 8-ketotrichothecenes 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol, fusarenon X and nivalenol. Food Chem Toxicol 50:2056–2061. doi:10.1016/j.fct.2012.03.055
Wu W, Bates MA, Bursian SJ et al (2013a) Peptide YY3-36 and 5-hydroxytryptamine mediate emesis induction by trichothecene deoxynivalenol (vomitoxin). Toxicol Sci 133:186–195. doi:10.1093/toxsci/kft033
Wu W, Bates MA, Bursian SJ et al (2013b) Comparison of emetic potencies of the 8-ketotrichothecenes deoxynivalenol, 15-acetyldeoxynivalenol, 3-acetyldeoxynivalenol, fusarenon X, and nivalenol. Toxicol Sci 131:279–291. doi:10.1093/toxsci/kfs286
Wu M, Xiao H, Ren W et al (2014a) Therapeutic effects of glutamic acid in piglets challenged with deoxynivalenol. PLoS One 9:e100591. doi:10.1371/journal.pone.0100591
Wu QH, Wang X, Yang W et al (2014b) Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: an update. Arch Toxicol 88:1309–1326. doi:10.1007/s00204-014-1280-0
Wu W, He K, Zhou HR et al (2014c) Effects of oral exposure to naturally-occurring and synthetic deoxynivalenol congeners on proinflammatory cytokine and chemokine mRNA expression in the mouse. Toxicol Appl Pharmacol. doi:10.1016/j.taap.2014.04.016
Wu W, Zhou H-R, He K et al (2014d) Role of cholecystokinin in anorexia induction following oral exposure to the 8-ketotrichothecenes deoxynivalenol, 15-acetyldeoxynivalenol, 3-acetyldeoxynivalenol, fusarenon X, and nivalenol. Toxicol Sci 138:278–289. doi:10.1093/toxsci/kft335
Wu L, Liao P, He L et al (2015) Growth performance, serum biochemical profile, jejunal morphology, and the expression of nutrients transporter genes in deoxynivalenol (DON)- challenged growing pigs. BMC Vet Res 11:144. doi:10.1186/s12917-015-0449-y
Wu W, Zhou H-R, Pestka JJ (2016) Potential roles for calcium-sensing receptor (CaSR) and transient receptor potential ankyrin-1 (TRPA1) in murine anorectic response to deoxynivalenol (vomitoxin). Arch Toxicol. doi:10.1007/s00204-016-1687-x
Xi P, Jiang Z, Dai Z et al (2011) Regulation of protein turnover by l-glutamine in porcine intestinal epithelial cells. J Nutr Biochem. doi:10.1016/j.jnutbio.2011.05.009
Yang GH, Jarvis BB, Chung YJ, Pestka JJ (2000) Apoptosis induction by the satratoxins and other trichothecene mycotoxins: relationship to ERK, p38 MAPK, and SAPK/JNK activation. Toxicol Appl Pharmacol 164:149–160. doi:10.1006/taap.1999.8888
Yang H, Chung DH, Kim YB et al (2008) Ribotoxic mycotoxin deoxynivalenol induces G(2)/M cell cycle arrest via p21(Cip/WAF1) mRNA stabilization in human epithelial cells. Toxicology 243:145–154. doi:10.1016/j.tox.2007.10.002
Yoshizawa T, Takeda H, Ohi T (1983) Structure of a novel metabolite from Deoxynivalenol, a trichothecene mycotoxin, in animals. Agric Biol Chem 47:2133–2135. doi:10.1080/00021369.1983.10865926
Young JC, Blackwell BA, ApSimon JW (1986) Alkaline degradation of the mycotoxin 4-deoxynivalenol. Tetrahedron Lett 27:1019–1022. doi:10.1016/S0040-4039(86)80037-5
Young JC, Zhou T, Yu H et al (2007) Degradation of trichothecene mycotoxins by chicken intestinal microbes. Food Chem Toxicol 45:136–143. doi:10.1016/j.fct.2006.07.028
Yu H, Zhou T, Gong J et al (2010) Isolation of deoxynivalenol-transforming bacteria from the chicken intestines using the approach of PCR-DGGE guided microbial selection. BMC Microbiol 10:182. doi:10.1186/1471-2180-10-182
Yunus AW, Ghareeb K, Twaruzek M et al (2012) Deoxynivalenol as a contaminant of broiler feed: effects on bird performance and response to common vaccines. Poult Sci 91:844–851. doi:10.3382/ps.2011-01873
Zhang X, Jiang L, Geng C et al (2009) The role of oxidative stress in deoxynivalenol-induced DNA damage in HepG2 cells. Toxicon 54:513–518. doi:10.1016/j.toxicon.2009.05.021
Zhou T, He J (2009) Bacterial isolate, methods of isolating bacterial isolates and Methods for detoxification of trichothecene mycotoxins. US Patent 61/249,023, 6 Oct 2009
Zhou HR, He K, Landgraf J et al (2014) Direct activation of ribosome-associated double-stranded RNA-dependent protein kinase (PKR) by deoxynivalenol, anisomycin and ricin: a new model for ribotoxic stress response induction. Toxins 6:3406–3425. doi:10.3390/toxins6123406
Acknowledgments
AP was supported by a CIFRE fellowship (2012/0572, jointly financed by the BIOMIN holding GmBH, ANRT and INRA). This work was supported in part by the ANR (Agence Nationale de la Recherche) projects ImBio (ANR-13-CESA-0003-03) and CaDON(ANR-15-CE21-0001-02) and the Partenariat Hubert Curien project (PHC 35813XM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Payros, D., Alassane-Kpembi, I., Pierron, A. et al. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch Toxicol 90, 2931–2957 (2016). https://doi.org/10.1007/s00204-016-1826-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1826-4