Abstract
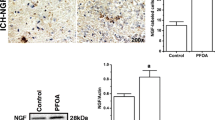
Perfluorooctane sulfonate (PFOS) is a persistent environmental contaminant. Although studies have described PFOS-induced neurotoxicity in animal brains and neuronal cells, the molecular mechanisms of PFOS-induced neurotoxicity based on the distribution properties, especially during developmental periods, have not been clarified. To clarify the mechanisms of PFOS-induced neuronal vulnerability during developmental periods, we examined changes in glutamate receptor 2 (GluR2) expression and related neurotoxicity in PFOS-treated primary cortical neurons and neonatal rat brains. Exposure of cortical neurons to 1 μM PFOS for 9 days resulted in decreased α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subunit GluR2 expression, which subsequently enhanced vulnerability to glutamate by increasing intracellular Ca2+ concentrations. The brain–plasma ratio of PFOS in pups was approximately five times higher than that in dams, although there were no differences in liver–plasma ratio between dams and pups. GluR2 expression in pup cerebral cortex decreased after exposure to 2.0 mg/kg PFOS, and kainic acid induced histopathological abnormalities in PFOS-exposed pups. Our findings suggest that decreased neuronal GluR2 expression is involved in PFOS-induced neurotoxicity, especially during the fetal and neonatal periods.





Similar content being viewed by others
References
Ambrosi G, Cerri S, Blandini F (2014) A further update on the role of excitotoxicity in the pathogenesis of Parkinson’s disease. J Neural Transm 121:849–859
Butenhoff JL, Olsen GW, Pfahles-Hutchens A (2006) The applicability of biomonitoring data for perfluorooctanesulfonate to the environmental public health continuum. Environ Health Perspect 114:1776–1782
Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL (2007) Polyfluoroalkyl chemicals in the U.S. Population: data from the national health and nutrition examination survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect 115:1596–1602
Chang SC, Ehresman DJ, Bjork JA, Wallace KB, Parker GA, Stump DG et al (2009) Gestational and lactational exposure to potassium perfluorooctanesulfonate (K + PFOS) in rats: toxicokinetics, thyroid hormone status, and related gene expression. Reprod Toxicol 27:387–399
Domingo JL (2012) Health risks of dietary exposure to perfluorinated compounds. Environ Int 40:187–195
Fei C, McLaughlin JK, Lipworth L, Olsen J (2009) Maternal levels of perfluorinated chemicals and subfecundity. Hum Reprod 24:1200–1205
Harada K, Xu F, Ono K, Iijima T, Koizumi A (2005) Effects of PFOS and PFOA on l-type Ca2+ currents in guinea-pig ventricular myocytes. Biochem Biophys Res Commun 329:487–494
Hollmann M, Heinemann S (1994) Cloned glutamate receptors. Annu Rev Neurosci 17:31–108
Houde M, De Silva AO, Muir DC, Letcher RJ (2011) Monitoring of perfluorinated compounds in aquatic biota: an updated review. Environ Sci Technol 45:7962–7973
Ishida K, Kotake Y, Miyara M, Aoki K, Sanoh S, Kanda Y et al (2013) Involvement of decreased glutamate receptor subunit GluR2 expression in lead-induced neuronal cell death. J Toxicol Sci 38:513–521
Isomura M, Kotake Y, Masuda K, Miyara M, Okuda K, Samizo S et al (2013) Tributyltin-induced endoplasmic reticulum stress and its Ca(2+)-mediated mechanism. Toxicol Appl Pharmacol 272:137–146
Johansson N, Fredriksson A, Eriksson P (2008) Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology 29:160–169
Johansson N, Eriksson P, Viberg H (2009) Neonatal exposure to PFOS and PFOA in mice results in changes in proteins which are important for neuronal growth and synaptogenesis in the developing brain. Toxicol Sci 108:412–418
Kannan K, Corsolini S, Falandysz J, Oehme G, Focardi S, Giesy JP (2002) Perfluorooctanesulfonate and related fluorinated hydrocarbons in marine mammals, fishes, and birds from coasts of the Baltic and the Mediterranean Seas. Environ Sci Technol 36:3210–3216
Kärrman A, Ericson I, van Bavel B, Darnerud PO, Aune M, Glynn A et al (2007) Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ Health Perspect 115:226–230
Kondo M, Sumino R, Okado H (1997) Combinations of AMPA receptor subunit expression in individual cortical neurons correlate with expression of specific calcium-binding proteins. J Neurosci 17:1570–1581
Kotake Y (2012) Molecular mechanisms of environmental organotin toxicity in mammals. Biol Pharm Bull 35:1876–1880
Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME et al (2003) Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol Sci 74:382–392
Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J (2007) Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99:366–394
Li SY, Jia YH, Sun WG, Tang Y, An GS, Ni JH et al (2010) Stabilization of mitochondrial function by tetramethylpyrazine protects against kainate-induced oxidative lesions in the rat hippocampus. Free Radic Biol Med 48:597–608
Liao CY, Li XY, Wu B, Duan S, Jiang GB (2008) Acute enhancement of synaptic transmission and chronic inhibition of synaptogenesis induced by perfluorooctane sulfonate through mediation of voltage-dependent calcium channel. Environ Sci Technol 42:5335–5341
Liu SJ, Zukin RS (2007) Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci 30:126–134
Long Y, Wang Y, Ji G, Yan L, Hu F, Gu A (2013) Neurotoxicity of perfluorooctane sulfonate to hippocampal cells in adult mice. PLoS one 8:e54176
Mariussen E (2012) Neurotoxic effects of perfluoroalkylated compounds: mechanisms of action and environmental relevance. Arch Toxicol 86:1349–1367
Nakatsu Y, Kotake Y, Komasaka K, Hakozaki H, Taguchi R, Kume T et al (2006a) Glutamate excitotoxicity is involved in cell death caused by tributyltin in cultured rat cortical neurons. Toxicol Sci 89:235–242
Nakatsu Y, Kotake Y, Ohta S (2006b) Tributyltin-induced cell death is mediated by calpain in PC12 cells. Neurotoxicology 27:587–593
Nakatsu Y, Kotake Y, Ohta S (2007) Concentration dependence of the mechanisms of tributyltin-induced apoptosis. Toxicol Sci 97:438–447
Nakatsu Y, Kotake Y, Takishita T, Ohta S (2009) Long-term exposure to endogenous levels of tributyltin decreases GluR2 expression and increases neuronal vulnerability to glutamate. Toxicol Appl Pharmacol 240:292–298
Oguro K, Oguro N, Kojima T, Grooms SY, Calderone A, Zheng X et al (1999) Knockdown of AMPA receptor GluR2 expression causes delayed neurodegeneration and increases damage by sublethal ischemia in hippocampal CA1 and CA3 neurons. J Neurosci 19:9218–9227
Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL et al (2007) Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115:1298–1305
Ong WY, Tanaka K, Dawe GS, Ittner LM, Farooqui AA (2013) Slow excitotoxicity in Alzheimer’s disease. J Alzheimers Dis 35:643–668
Seacat AM, Thomford PJ, Hansen KJ, Olsen GW, Case MT, Butenhoff JL (2002) Subchronic toxicity studies on perfluorooctanesulfonate potassium salt in cynomolgus monkeys. Toxicol Sci 68:249–264
Sepers MD, Raymond LA (2014) Mechanisms of synaptic dysfunction and excitotoxicity in Huntington’s disease. Drug Discov Today 19:990–996
Slotkin TA, MacKillop EA, Melnick RL, Thayer KA, Seidler FJ (2008) Developmental neurotoxicity of perfluorinated chemicals modeled in vitro. Environ Health Perspect 116:716–722
Sugiyama C, Kotake Y, Yamaguchi M, Umeda K, Tsuyama Y, Sanoh S et al (2015) Development of a simple measurement method for GluR2 protein expression as an index of neuronal vulnerability. Toxicol Rep 2:450–460
Tan DX, Manchester LC, Reiter RJ, Qi W, Kim SJ, El-Sokkary GH (1998) Melatonin protects hippocampal neurons in vivo against kainic acid-induced damage in mice. J Neurosci Res 54:382–389
Tang XJ, Xing F (2013) Calcium-permeable AMPA receptors in neonatal hypoxic-ischemic encephalopathy (review). Biomed Rep 1:828–832
Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Barbee BD, Richards JH et al (2003) Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: maternal and prenatal evaluations. Toxicol Sci 74:369–381
Zeng HC, Li YY, Zhang L, Wang YJ, Chen J, Xia W et al (2011) Prenatal exposure to perfluorooctanesulfonate in rat resulted in long-lasting changes of expression of synapsins and synaptophysin. Synapse 65:225–233
Acknowledgments
This work was supported by JSPS KAKENHI (B) Grant Numbers 23310047 (to Y. K.) and 15H02826 (to Y. K.) and Grant-in-Aid for JSPS Fellows Grant Number 14J06534 (to K. I.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ishida, K., Tsuyama, Y., Sanoh, S. et al. Perfluorooctane sulfonate induces neuronal vulnerability by decreasing GluR2 expression. Arch Toxicol 91, 885–895 (2017). https://doi.org/10.1007/s00204-016-1731-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1731-x