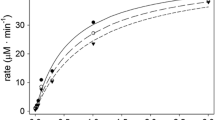
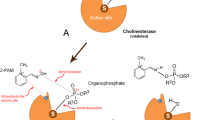
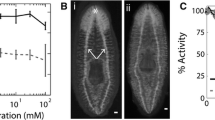
Abstract
Multiple epidemiological and experimental studies have demonstrated that exposure to organophosphorus compounds (OPs) is associated with a variety of neurological disorders. Some of these exposure symptoms cannot be precisely correlated with known molecular targets and mechanisms of toxicity. Most of the known molecular targets of OPs fall in the protein family of serine esterases. We have shown that three esterase components in the soluble fraction of chicken brain (an animal model frequently used in OP neurotoxicity assays) can be kinetically distinguished using paraoxon, mipafox and phenylmethyl sulfonyl fluoride as inhibitors, and phenyl valerate as a substrate; we termed them Eα, Eβ and Eγ. The Eα-component, which is highly sensitive to paraoxon and mipafox and resistant to PMSF, has shown sensitivity to the substrate acetylthiocholine, and to ethopropazine and iso-OMPA (specific inhibitors of butyrylcholinesterase; BChE) but not to BW 284C51 (a specific inhibitor of acetylcholinesterase; AChE). In this work, we employed a large-scale proteomic analysis B with a LC/MS/MS TripleTOF system; 259 proteins were identified in a chromatographic fractionated sample enriched in Eα activity of the chicken brain soluble fraction. Bioinformatics analysis revealed that BChE is the only candidate protein identified to be responsible for almost all the Eα activity. This study demonstrates the potential information to be gained from combining kinetic dissection with large-scale proteomics and bioinformatics analyses for identification of proteins that are targets of OP toxicity and may be involved in detoxification of phosphoryl and carbonyl esters.




Similar content being viewed by others
References
Bairoch A (1992) PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res 20(Suppl):2013–2018
Barril J, Estévez J, Escudero MA, Céspedes MV, Níguez N, Sogorb MA, Monroy A, Vilanova E (1999) Peripheral nerve soluble esterases are spontaneously reactivated after inhibition by paraoxon: implications for a new definition of neuropathy target esterase. Chem Biol Interact 119–120:541–550
Benabent M, Vilanova E, Mangas I, Sogorb MÁ, Estévez J (2014a) Interaction between substrates suggests a relationship between organophosphorus-sensitive phenylvalerate- and acetylcholine-hydrolyzing activities in chicken brain. Toxicol Lett 230(2):132–138
Benabent M, Vilanova E, Sogorb MÁ, Estévez J (2014b) Cholinesterase assay by an efficient fixed time endpoint method. MethodsX 1:258–263
Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL (2009) BLAST+: architecture and applications. BMC Bioinformatics 10:421
Carrington CD, Abou-Donia MB (1984) The correlation between the recovery rate of neurotoxic esterase activity and sensitivity to organophosphorus-induced delayed neurotoxicity. Toxicol Appl Pharmacol 75(2):350–357
Casida JE, Quistad GB (2005) Serine hydrolase targets of organophosphorus toxicants. Chem Biol Interact 157–158:277–283
Céspedes MV, Escudero MA, Barril J, Sogorb MA, Vicedo JL, Vilanova E (1997) Discrimination of carboxylesterases of chicken neural tissue by inhibition with a neuropathic, non-neuropathic organophosphorus compounds and neuropathy promoter. Chem Biol Interact 106(3):191–200
Chatonnet A, Lockridge O (1989) Comparison of butyrylcholinesterase and acetylcholinesterase. Biochem J 260(3):625–634
Chemnitius JM, Haselmeyer KH, Zech R (1983) Neurotoxic esterase. Identification of two isoenzymes in hen brain. Arch Toxicol 53(3):235–244
Costa LG (2006) Current issues in organophosphate toxicology. Clin Chim Acta 366(1–2):1–13
Cousin X, Hotelier T, Giles K, Lievin P, Toutant JP, Chatonnet A (1997) The alpha/beta fold family of proteins database and the cholinesterase gene server ESTHER. Nucleic Acids Res 25(1):143–146
Cucuianu M (1999) Serum gamma-glutamyltransferase and/or serum cholinesterase as markers of the metabolic syndrome. Diabetes Care 22(8):1381–1382
Cygler M, Schrag JD, Sussman JL, Harel M, Silman I, Gentry MK, Doctor BP (1993) Relationship between sequence conservation and three-dimensional structure in a large family of esterases, lipases, and related proteins. Protein Sci 2(3):366–382
Darvesh S, Walsh R, Kumar R, Caines A, Roberts S, Magee D, Rockwood K, Martin E (2003) Inhibition of human cholinesterases by drugs used to treat Alzheimer disease. Alzheimer Dis Assoc Disord 17(2):117–126
De Jaco A, Dubi N, Camp S, Taylor P (2012) Congenital hypothyroidism mutations affect common folding and trafficking in the α-hydrolase-fold proteins. FEBS J 279:4293–4305
Doorn JA, Schall M, Gage DA, Talley TT, Thompson CM, Richardson RJ (2001) Identification of butyrylcholinesterase adducts after inhibition with isomalathion using mass spectrometry: difference in mechanism between (1R)- and (1S)-stereoisomers. Toxicol Appl Pharmacol 176(2):73–80
Duysen EG, Li B, Lockridge O (2009) The butyrylcholinesterase knockout mouse are search tool in the study of drug sensitivity, bio-distribution, obesity and Alzheimer’s disease. Expert Opin Drug Metab Toxicol 5(5):523–528
Escudero MA, Céspedes MV, Vilanova E (1997) Chromatographic discrimination of soluble neuropathy target esterase isoenzymes and related phenyl valerate esterases from chicken brain, spinal cord, and sciatic nerve. J Neurochem 68(5):2170–2176
Estévez J, García-Pérez AG, Barril J, Pellín M, Vilanova E (2004) The inhibition of the high sensitive peripheral nerve soluble esterases by mipafox. A new mathematical processing for the kinetics of inhibition of esterases by organophosphorus compounds. Toxicol Lett 151(1):171–181
Estévez J, Barril J, Vilanova E (2010) Inhibition with spontaneous reactivation and the “ongoing inhibition” effect of esterases by biotinylated organophosphorus compounds: S9B as a model. Chem Biol Interact 187(1–3):397–402
Estévez J, García-Pérez A, Barril J, Vilanova E (2011) Inhibition with spontaneous reactivation of carboxyl esterases by organophosphorus compounds: paraoxon as a model. Chem Res Toxicol 24(1):135–143
Furlong CE (2011) Exposure to triaryl phosphates: metabolism and biomarkers of exposure. J Biol Phys Chem 11. doi:10.4024/28FU11A.jbpc.11.04
García-Pérez AG, Barril J, Estévez J, Vilanova E (2003) Properties of phenyl valerate esterase activities from chicken serum are comparable with soluble esterases of peripheral nerves in relation with organophosphorus compounds inhibition. Toxicol Lett 142(1–2):1–10
Giacobini E (2003) Cholinesterases: new roles in brain function and in Alzheimer's disease. Neurochem Res 28(3–4):515–522
Glynn P, Read DJ, Guo R, Wylie S, Johnson MK (1994) Synthesis and characterization of a biotinylated organophosphorus ester for detection and affinity purification of a brain serine esterase: neuropathy target esterase. Biochem J 301(Pt 2):551–556
Glynn P, Holton JL, Nolan CC, Read DJ, Brown L, Hubbard A, Cavanagh JB (1998) Neuropathy target esterase: immunolocalization to neuronal cell bodies and axons. Neurosci 83(1):295–302
Glynn P, Read DJ, Lush MJ, Li Y, Atkins J (1999) Molecular cloning of neuropathy target esterase (NTE). Chem Biol Interact 14(119–120):513–517
Grigoryan H, Schopfer LM, Peeples ES, Duysen EG, Grigoryan M, Thompson CM, Lockridge O (2009) Mass spectrometry identifies multiple organophosphorylated sites on tubulin. Toxicol Appl Pharmacol 240(2):149–158
Guttman M, Betts GN, Barnes H, Ghassemian M, van der Geer P, Komives EA (2009) Interactions of the NPXY microdomains of the low density lipoprotein receptor-related protein 1. Proteomics 9(22):5016–5028
Harel M, Sussman JL, Krejci E, Bon S, Chanal P, Massoulié J, Silman I (1992) Conversion of acetylcholinesterase to butyrylcholinesterase: modeling and mutagenesis. Proc Natl Acad Sci USA 89(22):10827–10831
Haux JE, Quistad GB, Casida JE (2000) Phosphobutyrylcholinesterase: phosphorylation of the esteratic site of butyrylcholinesterase by ethephon [(2-chloroethyl)phosphonic acid] dianion. Chem Res Toxicol 13(7):646–651
Hotelier T, Renault L, Cousin X, Negre V, Marchot P, Chatonnet A (2004) ESTHER, the database of the alpha/beta-hydrolase fold superfamily of proteins. Nucleic Acids Res 32((Database issue)):145–147
ICGSC (International Chicken Genome Sequencing Consortium) (2004) Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 432(7018):695–716
Jennings LL, Malecki M, Komives EA, Taylor P (2003) Direct analysis of the kinetic profiles of organophosphateacetylcholinesterase adducts by MALDI-TOF mass spectrometry. Biochemistry 42(37):11083–11091
Johnson MK (1975) Structure-activity relationships for substrates and inhibitors of hen brain neurotoxic esterase. Biochem Pharmacol 24(7):797–805
Johnson G, Moore SW (2012) Why has butyrylcholinesterase been retained? Structural and functional diversification in a duplicated gene. Neurochem Int 61(5):783–797
Kalow W, Gunn DR (1957) The relation between dose of succinylcholine and duration of apnea in man. J Pharmacol Exp Ther 120(2):203–214
Kalow W, Gunn DR (1959) Some statistical data on atypical cholinesterase of human serum. Ann Hum Genet 23:239–250
Kraut D, Goff H, Pai RK, Hosea NA, Silman I, Sussman JL, Taylor P, Voet JG (2000) Inactivation studies of acetylcholinesterase with phenylmethylsulfonyl fluoride. Mol Pharmacol 57(6):1243–1248
La Du BN, Bartels CF, Nogueira CP, Hajra A, Lightstone H, Van der Spek A, Lockridge O (1990) Phenotypic and molecular biological analysis of human butyrylcholinesterase variants. Clin Biochem 23(5):423–431
Lenfant N, Hotelier T, Bourne Y, Marchot P, Chatonnet A (2013) Proteins with an alpha/beta hydrolase fold: relationships between subfamilies in an ever-growing superfamily. Chem Biol Interact 203(1):266–268
Li B, Duysen EG, Saunders TL, Lockridge O (2006) Production of the butyrylcholinesterase knockout mouse. J Mol Neurosci 30(1–2):193–195
Lin D, Tabb DL, Yates JR 3rd (2003) Large-scale protein identification using mass spectrometry. Biochim Biophys Acta 1646(1–2):1–10
Lockridge O (1990) Genetic variants of human serum cholinesterase influence metabolism of the muscle relaxant succinylcholine. Pharmacol Ther 47(1):35–60
Lockridge O, Schopfer LM (2006) Biomarkers of exposure. In: Gupta RC (ed) Toxicology of organophosphate and carbamate compounds. Academic Press, San Diego, pp 703–715
Long JZ, Cravatt BF (2011) The metabolic serine hydrolases and their functions in mammalian physiology and disease. Chem Rev 111(10):6022–6063
Mackenzie Ross SJ, Brewin CR, Curran HV, Furlong CE, Abraham-Smith KM, Harrison V (2010) Neuropsychological and psychiatric functioning in sheep farmers exposed to low levels of organophosphate pesticides. Neurotoxicol Teratol 32(4):452–459
Mangas I, Vilanova E, Estévez J (2011) Kinetics of the inhibitory interaction of organophosphorus neuropathy inducers and non-inducers in soluble esterases in the avian nervous system. Toxicol Appl Pharmacol 256(3):360–368
Mangas I, Vilanova E, Estévez J (2012a) NTE and non-NTE esterases in brain membrane: kinetic characterization with organophosphates. Toxicology 297(1–3):17–25
Mangas I, Vilanova E, Estévez J (2012b) Phenylmethylsulfonyl fluoride, a potentiator of neuropathy, alters the interaction of organophosphorus compounds with soluble brain esterases. Chem Res Toxicol 25(11):2393–2401
Mangas I, Vilanova E, Benabent M, Estévez J (2014a) Separating esterase targets of organophosphorus compounds in the brain by preparative chromatography. Toxicol Lett 225(1):167–176
Mangas I, Vilanova E, Estévez J (2014b) Kinetic interactions of a neuropathy potentiator (phenylmethylsulfonyl fluoride) with the neuropathy target esterase and other membrane bound esterases. Arch Toxicol 88(2):355–366
Mangas I, Taylor P, Vilanova E, Estévez J, Franca TCC, Komives E, Radić Z (2015) Resolving pathways of interaction of mipafox and a sarin analog with human acetylcholinesterase by kinetics, mass spectrometry and molecular modeling approaches. Arch Toxicol. doi:10.1007/s00204-015-1481-1
Massoulié J (2002) The origin of the molecular diversity and functional anchoring of cholinesterases. Neurosignals 11(3):130–143
McCormack AL, Schieltz DM, Goode B, Yang S, Barnes G, Drubin D, Yates JR (1997) Direct analysis and identification of proteins in mixtures by LC/MS/MS and database searching at the low-femtomole level. Anal Chem 69(4):767–776
McDaniel KL, Moser VC (2004) Differential profiles of cholinesterase inhibition and neurobehavioral effects in rats exposed to fenamiphos or profenofos. Neurotoxicol Teratol 26(3):407–415
Myers A, Richmond RC, Oakeshott JG (1988) On the origins of esterases. Mol Biol Evol 5(2):113–119
Pope CN (1999) Organophosphorus pesticides: do they all have the same mechanism of toxicity? J Toxicol Environ Health B Crit Rev 2(2):161–181
Randell EW, Mathews MS, Zhang H, Seraj JS, Sun G (2005) Relationship between serum butyrylcholinesterase and the metabolic syndrome. Clin Biochem 38(9):799–805
Silman I, Sussman JL (2008) Acetylcholinesterase: how is structure related to function? Chem Biol Interact 175(1–3):3–10
Taylor P (2011) Anticholinesterase agents. In: Hardman JG, Limbird LE (eds) Goodman & Gilman’s the pharmacological basis of therapeutics, 12th edn. McGraw-Hill, New York, pp 175–191
Terry AV (2012) Functional consequences of repeated organophosphate exposure: potential non-cholinergic mechanisms. Pharmacol Ther 134(3):355–365
Valle AM, Radic Z, Rana BK, Mahboubi V, Wessel J, Shih PA, Rao F, O’Connor DT, Taylor P (2011) Naturally occurring variations in the human cholinesterase genes: heritability and association with cardiovascular and metabolic traits. J Pharmacol Exp Ther 338(1):125–133
Vilanova E, Barril J, Carrera V, Pellin MC (1990) Soluble and particulate forms of the organophosphorus neuropathy target esterase in hen sciatic nerve. J Neurochem 55(4):1258–1265
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mangas, I., Radić, Z., Taylor, P. et al. Butyrylcholinesterase identification in a phenylvalerate esterase-enriched fraction sensitive to low mipafox concentrations in chicken brain. Arch Toxicol 91, 909–919 (2017). https://doi.org/10.1007/s00204-016-1670-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1670-6