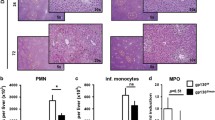
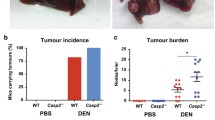
Abstract
Long-term exposure to carcinogens combined with chronic hepatitis contributes greatly to the worldwide high incidence of hepatocellular carcinoma (HCC). It is still unclear to which extent the release of pro-inflammatory reactive oxygen or nitrogen species contributes to the development of this malignancy. Here, we aim to elucidate the role of superoxide in a model of chemical hepatocarcinogenesis. p47phox knockout mice (KO), lacking superoxide formation by phagocytic NADPH oxidase (phox), and wild-type animals (WT) were subjected to two different initiation–promotion protocols: (1) single dose of diethylnitrosamine (DEN) at 6 weeks of age followed by phenobarbital (PB) via diet, or ethanol (EtOH) in drinking water; (2) DEN at neonatal age followed by three cytotoxic doses of DEN at intervals of 6–7 weeks. The appearance of tumors and prestages was quantified. There was no obvious difference in the capacity of DEN to initiate hepatocarcinogenesis in KO and WT mice. PB promoted tumor development in both genotypes without significant difference. EtOH induced steatosis significantly less in KO than in WT liver, but had no effect on tumor formation in either genotype. However, hepatocarcinogenesis by three cytotoxic DEN doses after neonatal initiation was attenuated significantly in KO. Macrophages/monocytes identified by the specific antigen F4/80 were more abundant in KO than in WT liver, possibly reflecting a compensatory response. We conclude that phox-derived superoxide is not essential but is supportive for the promotion of hepatocarcinogenesis by cytotoxic doses of DEN. The production of superoxide may therefore contribute to the promotion of hepatocarcinogenesis by cytotoxic/pro-inflammatory stimuli.





Similar content being viewed by others
Abbreviations
- BW:
-
Body weight
- DEN:
-
Diethylnitrosamine
- EtOH:
-
Ethanol
- Exp:
-
Experiment
- HCA:
-
Hepatocellular adenoma
- HCC:
-
Hepatocellular carcinoma
- KC:
-
Kupffer cell
- KO:
-
phox−/− mice
- n.s.:
-
Not significant
- MC:
-
Mesenchymal liver cells
- PB:
-
Phenobarbital
- PBS:
-
Phosphate-buffered saline
- phox:
-
Phagocytic NADPH oxidase
- phox−/−:
-
p47 Phagocytic NADPH oxidase deficient
- RLW:
-
Relative liver weight
- ROS:
-
Reactive oxygen species
- WT:
-
Wild-type mice
References
Ajakaiye M, Jacob A, Wu R, Nicastro JM, Coppa GF, Wang P (2011) Alcohol and hepatocyte-Kupffer cell interaction. Mol Med Rep 4(4):597–602
Bautista AP (1998) The role of Kupffer cells and reactive oxygen species in hepatic injury during acute and chronic alcohol intoxication. Alcohol Clin Exp Res 22(5 Suppl):255S–259S
Bedard K, Krause K-H (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87(1):245–313
Beland FA, Benson RW, Mellick PW, Kovatch RM, Roberts DW, Fang JL, Doerge DR (2005) Effect of ethanol on the tumorigenicity of urethane (ethyl carbamate) in B6C3F1 mice. Food Chem Toxicol 43(1):1–19
Böhm T, Berger H, Nejabat M, Riegler T, Kellner F, Kuttke M, Sagmeister S, Bazanella M, Stolze K, Daryabeigi A, Bintner N, Murkovic M, Wagner KH, Schulte-Hermann R, Rohr-Udilova N, Huber W, Grasl-Kraupp B (2013) Food-derived peroxidized fatty acids may trigger hepatic inflammation: a novel hypothesis to explain steatohepatitis. J Hepatol 59(3):563–570
Bonner MY, Arbiser JL (2012) Targeting NADPH oxidases for the treatment of cancer and inflammation. Cell Mol Life Sci 69(14):2435–2442
Bursch W, Chabicovsky M, Wastl U, Grasl-Kraupp B, Bukowska K, Taper H, Schulte-Hermann R (2005) Apoptosis in stages of mouse hepatocarcinogenesis: failure to counterbalance cell proliferation and to account for strain differences in tumor susceptibility. Toxicol Sci 85(1):515–529
Drucker C, Parzefall W, Teufelhofer O, Grusch M, Ellinger A, Schulte-Hermann R, Grasl-Kraupp B (2006) Non-parenchymal liver cells support the growth advantage in the first stages of hepatocarcinogenesis. Carcinogenesis 27(1):152–161
Duffy MJ, McKiernan E, O’Donovan N, McGowan PM (2009) The role of ADAMs in disease pathophysiology. Clin Chim Acta 403:31–36
Elcombe CR, Peffer RC, Wolf DC, Bailey J, Bars R, Bell D, Cattley RC, Ferguson SS, Geter D, Goetz A, Goodman JI, Hester S, Jacobs A, Omiecinski CJ, Schoeny R, Xie W, Lake BG (2014) Mode of action and human relevance analysis for nuclear receptor-mediated liver toxicity: a case study with phenobarbital as a model constitutive androstane receptor (CAR) activator. Crit Rev Toxicol 44(1):64–82
Enomoto N, Yamashina S, Kono H, Schemmer P, Rivera CA, Enomoto A, Nishiura T, Nishimura T, Brenner DA, Thurman RG (1999) Development of a new, simple rat model of early alcohol-induced liver injury based on sensitization of Kupffer cells. Hepatology 29(6):1680–1689
Forner A, Llovet JM, Bruix J (2012) Hepatocellular carcinoma. Lancet 379(9822):1245–1255
Freiler C, Brink A, Lutz WK, Kainzbauer E, Schulte-Hermann R, Parzefall W (2006) Hepatocarcinogenesis by diethylnitrosamine (DEN) in NADPH knock-out mice and their wild-type counterparts. Pharmacology 78:15
Freiler C, Kainzbauer E, Schulte-Hermann R, Parzefall W (2007) iNOS expression and levels of nitric oxide in a hepatocarcinogenesis model of p47-NADPH knockout mice. BMC Pharmacol 7(Suppl 2):A68
Grasl-Kraupp B, Luebeck G, Wagner A, Löw-Baselli A, de Gunst M, Waldhör T, Moolgavkar S, Schulte-Hermann R (2000) Quantitative analysis of tumor initiation in rat liver: role of cell replication and cell death (apoptosis). Carcinogenesis 21(7):1411–1421
Hanigan MH, Winkler ML, Drinkwater NR (1993) Induction of three histochemically distinct populations of hepatic foci in C57BL/6J mice. Carcinogenesis 14(5):1035–1040
Jackson SH, Gallin JI, Holland SM (1995) The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med 182:751–755
Jiang F, Zhang Y, Gregory J (2011) NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev 63:218–242
Katsuyama M (2010) Nox/Nadph oxidase, the superoxide-generating enzyme: its transcriptional regulation and physiological roles. J Pharmacol Sci 114:134–146
Kinoshita A, Wanibuchi H, Imaoka S, Ogawa M, Masuda C, Morimura K, Funae Y, Fukushima S (2002) Formation of 8-hydroxydeoxyguanosine and cell-cycle arrest in the rat liver via generation of oxidative stress by phenobarbital: association with expression profiles of p21(WAF1/Cip1), cyclin D1 and Ogg1. Carcinogenesis 23:341–349
Kono H, Rusyn I, Yin M, Gäbele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH, Bradford BU, Holland SM, Thurman RG (2000) NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest 106(7):867–872
Kraupp-Grasl B, Huber W, Putz B, Gerbracht U, Schulte-Hermann R (1990) Tumor promotion by the peroxisome proliferator nafenopin involving a specific subtype of altered foci in rat liver. Cancer Res 50(12):3701–3708
Kroll B, Kunz S, Klein T, Schwarz LR (1999) Effect of lindane and phenobarbital on cyclooxygenase-2 expression and prostanoid synthesis by Kupffer cells. Carcinogenesis 20(8):1411–1418
Kushida M, Wanibuchi H, Morimura K, Kinoshita A, Kang JS, Puatanachokchai R, Wei M, Funae Y, Fukushima S (2005) Dose-dependence of promotion of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline-induced rat hepatocarcinogenesis by ethanol: evidence for a threshold. Cancer Sci 96(11):747–757
Laskin DL, Robertson FM, Pilaro AM, Laskin JD (1988) Activation of liver macrophages following phenobarbital treatment of rats. Hepatology 8(5):1051–1055
Levin I, Petrasek J, Szabo G (2012) The presence of p47phox in liver parenchymal cells is a key mediator in the pathogenesis of alcoholic liver steatosis. Alcohol Clin Exp Res 36(8):1397–1406
Lieber CS, DeCarli LM (1989) Recommended amounts of nutrients do not abate the toxic effects of an alcohol dose that sustains significant blood levels of ethanol. J Nutr 119(12):2038–2040
Lloyd CM, Phillips AR, Cooper GJ, Dunbar PR (2008) Three-colour fluorescence immunohistochemistry reveals the diversity of cells staining for macrophage markers in murine spleen and liver. J Immunol Methods 334(1–2):70–81
Luo M, Yang F, Huang SX, Kuang ZP, Luo XL, Li YD, Wu JN, Xie YA (2013) Two-stage model of chemically induced hepatocellular carcinoma in mouse. Oncol Res 20(11):517–528
Macheiner D, Gauglhofer C, Rodgarkia-Dara C, Grusch M, Brachner A, Bichler C, Kandioler D, Sutterlüty H, Mikulits W, Schulte-Hermann R, Grasl-Kraupp B (2009) NORE1B is a putative tumor suppressor in hepatocarcinogenesis and may act via RASSF1A. Cancer Res 69(1):235–242
Mensinga TT, Speijers GJ, Meulenbelt J (2003) Health implications of exposure to environmental nitrogenous compounds. Toxicol Rev 22:41–51
Mochizuki Y, Furukawa K, Sawada N, Gotoh M (1981) Dose-dependent enhancing effect of phenobarbital on hepatocarcinogenesis initiated by diethylnitrosamine in the rat. Gann 72:170–173
Nalesnik MA, Michalopoulos GK (2012) Growth factor pathways in development and progression of hepatocellular carcinoma. Front Biosci 4:1487–1515
Peraino C, Fry RJM, Staffeldt E (1973) Enhancement of spontaneous hepatic tumorigenesis in C3H mice by dietary phenobarbital. J Natl Cancer lnst 57:1349–1351
Radike MJ, Stemmer KL, Bingham E (1981) Effect of ethanol on vinyl chloride carcinogenesis. Environ Health Perspect 41:59–62
Ross J, Plummer SM, Rode A, Scheer N, Bower CC, Vogel O, Henderson CJ, Wolf CR, Elcombe CR (2010) Human constitutive androstane receptor (CAR) and pregnane X receptor (PXR) support the hypertrophic but not the hyperplastic response to the murine nongenotoxic hepatocarcinogens phenobarbital and chlordane in vivo. Toxicol Sci 116(2):452–466
Sagmeister S, Drucker C, Losert A, Grusch M, Daryabeigi A, Parzefall W, Rohr-Udilova N, Bichler C, Smedsrød B, Kandioler D, Grünberger T, Wrba F, Schulte-Hermann R, Grasl-Kraupp B (2008) HB-EGF is a paracrine growth stimulator for early tumor prestages in inflammation-associated hepatocarcinogenesis. J Hepatol 49(6):955–964
Schulte-Hermann R, Bursch W, Grasl-Kraupp B (1995) Active cell death (apoptosis) in liver biology and disease. Prog Liver Dis 13:1–35
Seitz HK, Stickel F (2007) Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer 7(8):599–612
Spencer NY, Zhou W, Li Q, Zhang Y, Luo M, Yan Z, Lynch TJ, Abbott D, Banfi B, Engelhardt JF (2013) Hepatocytes produce tnfα following hypoxia/reoxygenation and liver ischemia/reperfusion in a nadph oxidase- and c-src-dependent manner. J Physiol Gastrointest Liver Physiol 305(1):G84–G94
Stickel F, Schuppan D, Hahn EG, Seitz HK (2002) Cocarcinogenic effects of alcohol in hepatocarcinogenesis. Gut 51(1):132–139
Tanaka T, Nishikawa A, Iwata H, Mori Y, Hara A, Hirono I, Mori H (1989) Enhancing effect of ethanol on aflatoxin B1-induced hepatocarcinogenesis in male ACI/N rats. Jpn J Cancer Res 80(6):526–530
Teufelhofer O, Parzefall W, Kainzbauer E, Ferk F, Freiler C, Knasmüller S, Elbling L, Thurman R, Schulte-Hermann R (2005) Superoxide generation from Kupffer cells contributes to hepatocarcinogenesis: studies on NADPH oxidase knockout mice. Carcinogenesis 26(2):319–329
Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE (2006) Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol 79(6):1348–1356
Thompson KJ, Swan RZ, Walling TL, Iannitti DA, McKillop IH, Sindram D (2013) Obesity, but not ethanol, promotes tumor incidence and progression in a mouse model of hepatocellular carcinoma in vivo. Surg Endosc 27(8):2782–2791
Tsuchishima M, George J, Shiroeda H, Arisawa T, Takegami T, Tsutsumi M (2013) Chronic ingestion of ethanol induces hepatocellular carcinoma in mice without additional hepatic insult. Dig Dis Sci 58(7):1923–1933
Tsukamoto H, French SW, Reidelberger RD, Largman C (1985) Cyclical pattern of blood alcohol levels during continuous intragastric ethanol infusion in rats. Alcohol Clin Exp Res 9(1):31–37
Weber E, Bannasch P (1994) Dose and time dependence of the cellular phenotype in rat hepatic preneoplasia and neoplasia induced by single oral exposures to N-nitrosomorpholine. Carcinogenesis 5(6):1219–1226
Wheeler MD, Kono H, Yin M, Nakagami M, Uesugi T, Arteel GE, Gäbele E, Rusyn I, Yamashina S, Froh M, Adachi Y, Iimuro Y, Bradford BU, Smutney OM, Connor HD, Mason RP, Goyert SM, Peters JM, Gonzalez FJ, Samulski RJ, Thurman RG (2001) The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic Biol Med 31(12):1544–1549
Acknowledgments
This project (“MARCAR”) received funding under European Union Innovative Medicine Initiative (IMI JU) under grant agreement Nr 115001. Funding by a grant from Herzfelder’sche Familienstiftung is gratefully acknowledged. The animal care was thoroughly performed by Dagmar Lehner and Kathi Bernscherer.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Parzefall, W., Freiler, C., Lorenz, O. et al. Superoxide deficiency attenuates promotion of hepatocarcinogenesis by cytotoxicity in NADPH oxidase knockout mice. Arch Toxicol 89, 1383–1393 (2015). https://doi.org/10.1007/s00204-014-1298-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-014-1298-3