Abstract
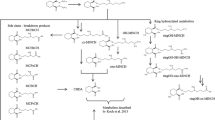
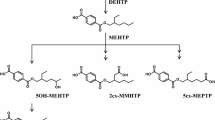
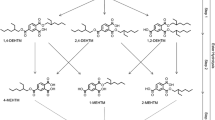
Hexamoll® DINCH® (diisononyl-cyclohexane-1,2-dicarboxylate) is a new high-molecular-weight plasticizer and a phthalate substitute. In this study, the metabolism of DINCH® was investigated by oral dosage of three male volunteers with approximately 50 mg Hexamoll® DINCH® (resulting in individual doses between 0.552 and 0.606 mg/kg body weight). Their urine samples were consecutively collected over 48 h. In analogy to di-iso-nonylphthalate (DINP) metabolism, we quantified the simple monoester mono-isononyl-cyclohexane-1,2-dicarboxylate (MINCH) and its secondary oxidized metabolites with HPLC–MS/MS via isotope dilution analysis. Additionally, we quantified the unspecific full breakdown product, cyclohexane-1,2-dicarboxylic acid (CHDA), via standard addition. All postulated metabolites were present in all samples analyzed. The unspecific CHDA was identified as the major urinary metabolite representing 23.7 % of the dose as the mean of the three volunteers (range 20.0–26.5 %). 14.8 % (11.3–16.7 %) of the dose was excreted as monoesters with oxidative modifications, in particular OH-MINCH 10.7 % (7.7–12.9 %), oxo-MINCH 2.0 % (1.5–2.6 %) and carboxy-MINCH 2.0 % (1.8–2.3 %). Less than 1 % was excreted as the simple monoester MINCH. In sum, 39.2 % (35.9–42.4 %) of the DINCH® dose was excreted as these metabolites in urine within 48 h. Over 90 % of the metabolites investigated were excreted within 24 h after application. The secondary oxidized metabolites, with elimination half-times between 10 and 18 h, proved to be apt and specific biomarkers to determine DINCH® exposure. With this study, we provide reliable urinary excretion factors to calculate DINCH® intakes based on these metabolites in environmental and occupational studies.




Similar content being viewed by others
References
Clark B, Smith DA (eds) (1986) An introduction to pharmacokinetics, 2nd edn. Blackwell Scientific, Oxford
Consumer Product Safety Commission’s (2010) Toxicity review for diisononyl phthalate DINP, http://www.cpsc.gov/about/cpsia/toxicityDINP.pdf. Accessed 18 Jul 2012
Crespo JE, Balart R, Sanchez L, Lopez J (2007) Substitution of di(2-ethylhexyl) phthalate by di(isononyl) cyclohexane-1,2-dicarboxylate as a plasticizer for industrial vinyl plastisol formulations. J Appl Polym Sci 104(2):1215–1220
Crespo JE, Sanchez L, Balart R, Lopez J (2009) Mechanical properties and fracture surface morphology of dehp and DINCH based vinyl plastisols. J Elastomers Plast 41(2):145–161
Directive 2005/84/EC of the European Parliament and of the Council (2005) Vol 344, Official Journal of the European Union. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2005:344:0040:0043:EN:PDF. Accessed 15 Jul 2012
EFSA (2006) Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request related to a 12th list of substances for food contact materials. EFSA J 395–401:1–21
European Chemicals Bureau (2008) European union risk assessment report bis(2-ethylhexyl)phthalate (DEHP) http://publications.jrc.ec.europa.eu/repository/bitstream/111111111/5648/1/dehpreport042.pdf. Accessed 18 July 2012
European Council for Plasticisers and Intermediates (2009) Review of recent scientific data on Di-isononyl Phthalate (DINP) and risk characterisation for its use in toys and childcare articles. http://www.cpsc.gov/about/cpsia/docs/DINPToysExxon062009.pdf. Accessed 6 Aug 2012
Koch HM, Angerer J (2007) Di-iso-nonylphthalate (DINP) metabolites in human urine after a single oral dose of deuterium-labelled DINP. Int J Hyg Environ Health 210(1):9–19
Koch HM, Drexler H, Angerer J (2003a) An estimation of the daily intake of di(2-ethylhexyl)phthalate (DEHP) and other phthalates in the general population. Int J Hyg Environ Health 206(2):77–83
Koch HM, Gonzalez-Reche LM, Angerer J (2003b) On-line clean-up by multidimensional liquid chromatography-electrospray ionization tandem mass spectrometry for high throughput quantification of primary and secondary phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci 784(1):169–182
Koch HM, Bolt HM, Angerer J (2004) Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch Toxicol 78(3):123–130. doi:10.1007/s00204-003-0522-3
Koch HM, Preuss R, Angerer J (2006) Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure- an update and latest results. Int J Androl 29(1):155–165
Koch HM, Muller J, Angerer J (2007) Determination of secondary, oxidised di-iso-nonylphthalate (DINP) metabolites in human urine representative for the exposure to commercial DINP plasticizers. J Chromatogr B Analyt Technol Biomed Life Sci 847(2):114–125
Lorber M, Koch HM, Angerer J (2010) A critical evaluation of the creatinine correction approach: can it underestimate intakes of phthalates? A case study with di-2-ethylhexyl phthalate. J Expos Sci Environ Epidemiol 21(6):576–586. doi:10.1038/jes.2010.43
National Industrial Chemicals Notification and Assessment Scheme (2008) 1,2-Cyclohexanedicarboxylic acid, 1,2-diisononyl ester (‘Hexamoll DINCH’). http://www.nicnas.gov.au/publications/car/new/std/stdfullr/std1000fr/std1259fr.pdf. Accessed 12 Oct 2011
Patent WO 99/32427 (1999) Verfahren zur Hydrierung von Benzolpolycarbonsäuren oder Derivaten davon unter Verwendung eines Makroporen aufweisenden Katalysators. BASF A.G
Regulation (EC) No. 1907/2006 of the European Parliament and of the Council (2006) Official Journal of the European Union http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=oj:l:2006:396:0001:0849:en:pdf. Accessed 6 Aug 2012
Rothenbacher T, Schwack W (2009) Rapid and nondestructive analysis of phthalic acid esters in toys made of poly(vinyl chloride) by direct analysis in real time single-quadrupole mass spectrometry. Rapid Commun Mass Spectrom 23(17):2829–2835
Schütze A, Pälmke C, Angerer J, Weiss T, Brüning T, Koch HM (2012) Quantification of biomarkers of environmental exposure to di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH) in urine via HPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 895–896:123–130. doi:10.1016/j.jchromb.2012.03.030
Silva MJ, Barr DB, Reidy JA, Kato K, Malek NA, Hodge CC, Hurtz D, Calafat AM, Needham LL, Brock JW (2003) Glucuronidation patterns of common urinary and serum monoester phthalate metabolites. Arch Toxicol 77(10):561–567. doi:10.1007/s00204-003-0486-3
Silva MJ, Furr J, Preau JLJ, Samandar E, Earl Gray L, Calafat AM (2012) Identification of potential biomarkers of exposure to di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH), an alternative for phthalate plasticizers. J Expo Sci Environ Epidemiol 22(2):204–211. doi:10.1038/jes.2011.43
Wang RY, Wan CY, Wang SF, Zhang Y (2009) Morphology, mechanical properties, and durability of Poly(Lactic Acid) plasticized with di(Isononyl) Cyclohexane-1,2-Dicarboxylate. Polym Eng Sci 49(12):2414–2420
Acknowledgments
The study was performed under the framework of the cooperation project of the German Federal Environment Ministry (BMU) and German Chemicals Industry Association (VCI) on human biomonitoring in order to improve the knowledge of substances taken up by the human organism. The metabolism study and method development were financed by the Chemie Wirtschaftsförderungsgesellschaft mbH. We would like to thank Dr. Rainer Otter, BASF SE, for excellent support within the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koch, H.M., Schütze, A., Pälmke, C. et al. Metabolism of the plasticizer and phthalate substitute diisononyl-cyclohexane-1,2-dicarboxylate (DINCH®) in humans after single oral doses. Arch Toxicol 87, 799–806 (2013). https://doi.org/10.1007/s00204-012-0990-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-012-0990-4