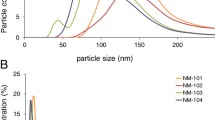
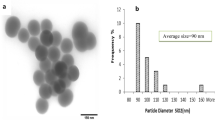
Abstract
The tissue distribution and toxicity of intravenously administered nanoparticles of titanium dioxide (TiO2) (>10 wt.% at <100 nm size) were investigated because of the fundamental importance to obtain information on the kinetics of this widely used nanoparticle in a situation of 100% bioavailability. Male Wistar rats were treated with single intravenous injections of a suspension of TiO2 in serum (5 mg/kg body weight), and the tissue content of TiO2 was determined 1, 14, and 28 days later. Biochemical parameters and antigens in serum were also assessed to determine potential pathological changes. The health and behavior of the animals were normal throughout the study. There were no detectable levels of TiO2 in blood cells, plasma, brain, or lymph nodes. The TiO2 levels were highest in the liver, followed in decreasing order by the levels in the spleen, lung, and kidney, and highest on day 1 in all organs. TiO2 levels were retained in the liver for 28 days, there was a slight decrease in TiO2 levels from day 1 to days 14 and 28 in the spleen, and a return to control levels by day 14 in the lung and kidney. There were no changes in the cytokines and enzymes measured in blood samples, indicating that there was no detectable inflammatory response or organ toxicity. Overall, rats exposed to TiO2 nanoparticles by a route that allows immediate systemic availability showed expected tissue distribution, no obvious toxic health effects, no immune response, and no change in organ function. Therefore, even with 100% bioavailability of the 5 mg/kg TiO2 dose afforded by the intravenous route of administration, there were no remarkable toxic effects evident in the experimental animals. These results indicate that TiO2 nanoparticles could be used safely in low doses.





Similar content being viewed by others
References
Bermudez E, Mangum J, Wong B, Asgharian B, Hext P, Warheit D, Everitt JI (2004) Pulmonary responses of mice, rats, and Hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol Sci 77:347–357
Boffetta P, Soutar A, Cherrie JW, Granath F, Anderson A, Anttila A, Blettner M, Gaborieau V, Klug SJ, Langard S, Luce D, Merletti F, Miller B, Mirabelli D, Pukkala E, Adami HO, Weiderpass E (2004) Mortality among workers employed in the titanium dioxide production industry in Europe. Cancer Causes Control 15:697–706
Chen JL, Fayerweather WE (1988) Epidemiologic study of workers exposed to titanium dioxide. J Occup Med 30:937–942
Cölfen H (2004) Analytical ultracentrifugation of nanoparticles. In: Nalwa HS (ed) Encyclopedia of Nanoscience and Nanotechnology. American Scientific Publishers, Valencia, CA, pp 67–88
EC Commission Directive 87/302/EEC of November 18, 1987; Part B: Methods for the determination of toxicity (1988). Toxicokinetics; Official Journal of the European Communities No. L 133:51-54
ECETOC (2006) Workshop on testing strategies to establish the safety of Nanomaterials 7–8 November 2005, Barcelona, Workshop Report No. 7. ECETOC, Brussels, Belgium
Grassian VH, O’Shaughnessy PT, Adamcakova-Dodd A, Pettibone JM, Thorne PS (2007) Inhalation exposure study of titanium dioxide nanoparticles with a primary size of 2 to 5 nm. Environ Health Perspect 115(3):397–402
Huggins CB (1939) A quantitative study of the activity of the reticuloendothelial structures in bone marrow in normal and ischemic limbs as indicated by India ink and titanium dioxide. Anat Rec 74:231
Huggins CB, Froehlich JP (1966) High concentration of injected titanium dioxide in abdominal lymph nodes. J Exp Med 124(6):1099–1106
Japan/MAFF: guidelines on the Compiling of Test Results on Toxicity; Tests on In Vivo Fate In Animals (2001)
Kragh-Hansen U (1990) Dan Med Bull 37:57
Lin MY, Lindsay HM, Weitz DA, Ball RC, Klein R, Meakin P (1990) Universal reaction-limited colloid aggregation. Phys Rev A 41:2005–2020
Mächtle W, Börger L (2006) Analytical Ultracentrifugation of polymers and Nanoparticles. Springer, Berlin
Ma-Hock L, Gamer AO, Landsiedel R, Leibold E, Frechen T, Sens B, Linsenbuehler M, van Ravenzwaay B (2007) Generation and characterization of test atmospheres with nanomaterials. Inhal Toxicol 19:833–848. doi:10.1080/08958370701479190
Oberdörster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K et al (2005) Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Particle Fibre Toxicol. doi:10.1186/1743-8977-2-8 [Online 6 October 2005]
OECD Guidelines for Testing of Chemicals (1984) Method No. 417 Toxicokinetics, Version dated 4.4.1984
Ross R (2007) Clearing the air. Town Ctry Mag April: 68
Umbreit T, Weaver JL, Miller TJ, Zhang J, Shah R, Stratmeyer ME, Tomazic-Jezic V (2007) Toxicology of titanium dioxide (TiO2) nanoparticles: 1. Characterization and tissue distribution in subcutaneously and intravenously injected mice. Toxicologist 96(1):287. Society of Toxicology 46th Annual Meeting and ToxExpo. Abstract #1386. March 25–29, 2007, Charlotte, NC
U.S. EPA, Health Effects Guidelines, OPPTS 870.7485, Metabolism and Pharmacokinetics, August (1998). http://www.epa.gov/opptstrs/publications/oppts_Harmonized/S70_Health_Effects_Test_Guidelines/Series/S70-7485.pdf
Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, Jia G, Gao Y, Li B, Sun J, Li Y, Jiao F, Zhao Y, Chai Z (2007) Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett 168(2):176–185
Warheit DB, Webba TR, Reeda KL, Frerichsb S, Sayesa CM (2007) Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: differential responses related to surface properties. Toxicology 230(1):90–104
Weaver J, Umbreit TH, Miller TJ, Zhang J, Stratmeyer ME, Tomazic-Jezic V (2007) Toxicology of titanium dioxide (TiO2) nanoparticles: 2. Immunological effects in subcutaneously and intravenously injected mice. Toxicologist 96(1):288. Society of Toxicology 46th Annual Meeting and ToxExpo. Abstract #1391, March 25–29, 2007, Charlotte, NC
Zhang HZ, Penn RL, Hamers RJ, Banfield JF (1999) Enhanced adsorption of molecules on surfaces of nanocrystalline particles. J Phys Chem B 103:4656–4662
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fabian, E., Landsiedel, R., Ma-Hock, L. et al. Tissue distribution and toxicity of intravenously administered titanium dioxide nanoparticles in rats. Arch Toxicol 82, 151–157 (2008). https://doi.org/10.1007/s00204-007-0253-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-007-0253-y