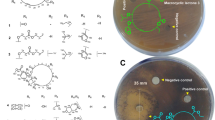
Abstract
Infectious diseases threaten global health due to the ability of microbes to acquire resistance against clinically used antibiotics. Continuous discovery of antibiotics with a novel mode of action is thus required. Actinomycetes and fungi are currently the major sources of antibiotics, but the decreasing rate of discovery of novel antibiotics suggests that the focus should be changed to previously untapped groups of microbes. Lysobacter species have a genome size of ~6 Mb with a relatively high G + C content of 61–70 % and are characterized by their ability to produce peptides that damage the cell walls or membranes of other microbes. Genome sequence analysis revealed that each Lysobacter species has gene clusters for the production of 12–16 secondary metabolites, most of which are peptides, thus making them ‘peptide production specialists’. Given that the number of antibiotics isolated is much lower than the number of gene clusters harbored, further intensive studies of Lysobacter are likely to unearth novel antibiotics with profound biomedical applications. In this review, we summarize the structural diversity, activity and biosynthesis of lysobacterial antibiotics and highlight the importance of Lysobacter species for antibiotic production.




Similar content being viewed by others
References
Berdy J (2005) Bioactive microbial metabolites. J Antibiot 58:1–26
Chen H, Olson AS, Su W, Dussault PH, Du L (2015) Fatty acyl incorporation in the biosynthesis of WAP-8294A, a group of potent anti-MRSA cyclic lipodepsipeptides. RSC Adv 5:105753–105759
Christensen P, Cook FD (1978) Lysobacter, a new genus of nonfruiting, gliding bacteria with a high base ratio. Int J Syst Evol Microbiol 28:367–393
Darling AE, Mau B, Perna NT (2010) progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 5:e11147
de Bruijn I et al (2015) Comparative genomics and metabolic profiling of the genus Lysobacter. BMC Genom 16:1–16
Demirev AV, Lee C-H, Jaishy BP, Nam D-H, Ryu DDY (2006) Substrate specificity of nonribosomal peptide synthetase modules responsible for the biosynthesis of the oligopeptide moiety of cephabacin in Lysobacter lactamgenus. FEMS Microbiol Lett 255:121–128
Ding Y et al (2016a) Alteramide B is a microtubule antagonist of inhibiting Candida albicans. Biochim Biophys Acta 1860:2097–2106
Ding Y et al (2016b) HSAF-induced antifungal effects in Candida albicans through ROS-mediated apoptosis. RSC Adv 6:30895–30904
Graupner PR et al (1997) Dihydromaltophilin; a novel fungicidal tetramic acid containing metabolite from Streptomyces sp. J Antibiot 50:1014–1019
Hamamoto H et al (2015) Lysocin E is a new antibiotic that targets menaquinone in the bacterial membrane. Nat Chem Biol 11:127–133
Harada S, Tsubotani S, Ono H, Okazaki H (1984) Cephabacins, new cephem antibiotics of bacterial origin. II. Isolation and characterization. J Antibiot 37:1536–1545
Harada S, Tsubotani S, Hida T, Ono H, Okazaki H (1986) Structure of lactivicin, an antibiotic having a new nucleus and similar biological activities to β-lactam antibiotics. Tetrahedron Lett 27:6229–6232
Hashizume H, Igarashi M, Hattori S, Hori M, Hamada M, Takeuchi T (2001) Tripropeptins, novel antimicrobial agents produced by Lysobacter sp. I. Taxonomy, isolation and biological activities. J Antibiot 54:1054–1059
Hashizume H, Hattori S, Igarashi M, Akamatsu Y (2004) Tripropeptin E, a new tripropeptin group antibiotic produced by Lysobacter sp. BMK333-48F3. J Antibiot 57:394–399
Hashizume H, Igarashi M, Sawa R, Adachi H, Nishimura Y, Akamatsu Y (2008) A new type of tripropeptin with anteiso-branched chain fatty acid from Lysobacter sp. BMK333-48F3. J Antibiot 61:577–582
Hashizume H et al (2011) Tripropeptin C blocks the lipid cycle of cell wall biosynthesis by complex formation with undecaprenyl pyrophosphate. Antimicrob Agents Chemother 55:3821–3828
Hashizume H, Takahashi Y, Harada S, Nomoto A (2015) Natural lipopeptide antibiotic tripropeptin C revitalizes and synergistically potentiates the activity of beta-lactams against methicillin-resistant Staphylococcus aureus. J Antibiot 68:373–378
Hou J, Robbel L, Marahiel Mohamed A (2011) Identification and characterization of the lysobactin biosynthetic gene cluster reveals mechanistic insights into an unusual termination module architecture. Chem Biol 18:655–664
Kato A et al (1998) A new anti-MRSA antibiotic complex, WAP-8294A. I. Taxonomy, isolation and biological activities. J Antibiot 51:929–935
Komatsu M, Uchiyama T, Omura S, Cane DE, Ikeda H (2010) Genome-minimized Streptomyces host for the heterologous expression of secondary metabolism. Proc Natl Acad Sci USA 107:2646–2651
Korp J, Gurovic MSV, Nett M (2016) Antibiotics from predatory bacteria. Beilstein J Org Chem 12:594–607
Laureti L et al (2011) Identification of a bioactive 51-membered macrolide complex by activation of a silent polyketide synthase in Streptomyces ambofaciens. Proc Natl Acad Sci USA 108:6258–6263
Lee W et al (2016) The mechanism of action of lysobactin. J Am Chem Soc 138:100–103
Li Y et al (2014) Iterative assembly of two separate polyketide chains by the same single-module bacterial polyketide synthase in the biosynthesis of HSAF. Angew Chem Int Ed 53:7524–7530
Lou L et al (2011) Biosynthesis of HSAF, a tetramic acid-containing macrolactam from Lysobacter enzymogenes. J Am Chem Soc 133:643–645
Lucas X et al (2013) StreptomeDB: a resource for natural compounds isolated from Streptomyces species. Nucleic Acids Res 41:D1130–D1136
Medema MH et al (2011) antiSMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res 39:W339–W346
Meyers E et al (1985) Catacandins, novel anticandidal antibiotics of bacterial origin. J Antibiot 38:1642–1648
Nett M, Ikeda H, Moore BS (2009) Genomic basis for natural product biosynthetic diversity in the actinomycetes. Nat Prod Rep 26:1362–1384
Nozaki Y et al (1984) Cephabacins, new cephem antibiotics of bacterial origin. IV. Antibacterial activities, stability to beta-lactamases and mode of action. J Antibiot 37:1555–1565
O’Sullivan J, McCullough JE, Tymiak AA, Kirsch DR, Trejo WH, Principe PA (1988) Lysobactin, a novel antibacterial agent produced by Lysobacter sp. I. Taxonomy, isolation and partial characterization. J Antibiot 41:1740–1744
Panthee S et al (2011) Furaquinocins I and J: novel polyketide isoprenoid hybrid compounds from Streptomyces reveromyceticus SN-593. J Antibiot 64:509–513
Panthee S, Hamamoto H, Suzuki Y, Sekimizu K (2016) In silico identification of lysocin biosynthetic gene cluster from Lysobacter sp. RH2180-5. J Antibiot. doi:10.1038/ja.2016.102
Paudel A, Hamamoto H, Panthee S, Sekimizu K (2016) Menaquinone as a potential target of antibacterial agents. Drug Discov Ther 10:123–128
Seccareccia I, Kost C, Nett M (2015) Quantitative analysis of Lysobacter predation. Appl Environ Microbiol 81:7098–7105
Shoji J et al (1988) Isolation and characterization of katanosins A and B. J Antibiot 41:713–718
von Nussbaum F et al (2007) Structure and total synthesis of lysobactin (katanosin B). Angew Chem Int Ed 46:2039–2042
Waksman SA (1945) Microbial antagonisms and antibiotic substances. The Commonwealth Fund, New York
Wang Y, Qian G, Liu F, Li Y-Z, Shen Y, Du L (2013) Facile method for site-specific gene integration in Lysobacter enzymogenes for yield improvement of the anti-MRSA antibiotics WAP-8294A and the antifungal antibiotic HSAF. ACS Synth Biol 2:670–678
Wang Y et al (2014) Transcriptomic analysis reveals new regulatory roles of Clp signaling in secondary metabolite biosynthesis and surface motility in Lysobacter enzymogenes OH11. Appl Microbiol Biotechnol 98:9009–9020
WHO (2014) Antimicrobial resistance: global report on surveillance. World Health Organization, Geneva, Switzerland
Xie Y, Wright S, Shen Y, Du L (2012) Bioactive natural products from Lysobacter. Nat Prod Rep 29:1277–1287
Xu G, Zhao Y, Du L, Qian G, Liu F (2015a) Hfq regulates antibacterial antibiotic biosynthesis and extracellular lytic-enzyme production in Lysobacter enzymogenes OH11. Microb Biotechnol 8:499–509
Xu L, Wu P, Wright SJ, Du L, Wei X (2015b) Bioactive polycyclic tetramate macrolactams from Lysobacter enzymogenes and their absolute configurations by theoretical ECD calculations. J Nat Prod 78:1841–1847
Yu F et al (2007) Structure and biosynthesis of heat-stable antifungal factor (HSAF), a broad-spectrum antimycotic with a novel mode of action. Antimicrob Agents Chemother 51:64–72
Zhang W et al (2011) Identification and characterization of the anti-methicillin-resistant Staphylococcus aureus WAP-8294A2 biosynthetic gene cluster from Lysobacter enzymogenes OH11. Antimicrob Agents Chemother 55:5581–5589
Acknowledgments
Lysocin research in our laboratory was supported by MEXT KAKENHI (JP221S0002), Grant-in-Aid for Scientific Research on Innovative Areas (JP26102714) and a Grant-in-Aid for young scientists (A) (JP24689008) to HH; and in part by Grant-in-Aid for scientific research (S) (JP15H05783) and Drug Discovery Support Promotion Project from Japan Agency for Medical Research and Development, AMED, to K.S.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Markus Nett.
Rights and permissions
About this article
Cite this article
Panthee, S., Hamamoto, H., Paudel, A. et al. Lysobacter species: a potential source of novel antibiotics. Arch Microbiol 198, 839–845 (2016). https://doi.org/10.1007/s00203-016-1278-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-016-1278-5