Abstract
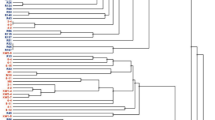
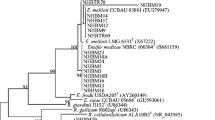
Forty-six Mesorhizobium strains associated with the leguminous plants Leucaena leucocephala and Sesbania herbacea in an uncultivated Mexican field were characterized using a polyphasic approach. The strains were identified as Mesorhizobium plurifarium based upon the close relationships with the reference strains for this species in PCR-based restriction fragment length polymorphism analyses, sequencing of 16S rRNA genes, multilocus enzyme electrophoresis, and DNA-DNA hybridization. Although the strains isolated from both plants formed the same group in multilocus enzyme electrophoresis and cross-nodulations were observed in the laboratory, different electrophoretic types were obtained from the two plants grown in natural soils, indicating the existence of a preferable association between the plants and the rhizobia. The M. plurifarium strains from Mexico and the reference strains from Africa and Brazil formed different phenotypic clusters in a numerical taxonomy. The Mexican strains did not grow at 37 °C and were sensitive to salty-alkaline conditions, while the reference strains from Africa and Brazil grew at 42 °C and were more resistant to salty-alkaline conditions. These results demonstrate that both the plants and environmental factors affected the evolution of rhizobia and that the Mexican strains had adapted to the neutral soils and the cool climate where they were isolated.



Similar content being viewed by others
References
Becker M, Ladha JK, Ottow JCG (1990) Growth and nitrogen fixation of two stem-nodulating legumes and their effects as green manure on lowland rice. Soil Biol Biochem 22:1109–1119
Bergersen FJ (1961) The growth of Rhizobium in synthetic media. Aust J Biol 14:349–360
Brenner DJ, Staley JT, Krieg NR. (2001) Classification of procaryotic organisms and the concept of bacterial speciation. In: Boone DR, Castenholz RW, Garrity GM (eds) Bergey’s manual of systematic bacteriology, 2nd edn, vol 1. Springer, Berlin Heidelberg New York pp 27–31
Caballero-Mellado J, Martínez-Romero E (1994) Limited genetic diversity in the endophytic sugarcane bacterium Acetobacter diazotrophicus. Appl Environ Microbiol 60:1532–1537
Caballero-Mellado J, Martínez-Romero E (1999) Soil fertilizer limits the genetic diversity of Rhizobium in bean nodules. Symbiosis 26:111–121
Chen WX, Li GS, Qi YL, Wang ET, Yuan HL, Li JL (1991) Rhizobium huakuii sp. nov. isolated from the root nodules of Astragalus sinicus. Int J Syst Bacteriol 41:275–280
Chen WX, Wang ET, Wang SY, Li YB, Chen XQ, Li Y (1995) Characteristics of Rhizobium tianshanense sp. nov., a moderately and slowly growing root nodule bacterium isolated from an arid saline environment in Xinjiang, People’s Republic of China. Int J Syst Bacteriol 45:153–159
De Lajudie P, Willems A, Nick G, Moreira F, Molouba F, Hoste B, Torck U, Neyra M, Collins MD, Lindström K, Dreyfus B, Gillis M (1998) Characterization of tropical tree rhizobia and description of Mesorhizobium plurifarium sp. nov. Int J Syst Bacteriol 48:369–382
De Ley J (1970) Reexamination of the association between melting point, buoyant density, and chemical base composition of deoxyribonucleic acid. J Bacteriol 101:738–754
Dröge M, Pühler A, Selbitschka W (1999) Horizontal gene transfer among bacteria in terrestrial and aquatic habitats as assessed by microcosm and field stidies. Biol Fertil Soils 29:221–245
Eckhardt T (1978) A rapid method for the identification of plasmid deoxyribonucleic acid in bacteria. Plasmid 1:584–588
Fahraeus G (1957) The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol 16:374–381
Gao JL, Sun JG, Li Y, Wang ET, Chen WX (1994) Numerical taxonomy and DNA relatedness of tropical rhizobia isolated from Hainan Province, China. Int J Syst Bacteriol 44:151–158
Genetics Computer Group. (1995). Program manual for the Wisconsin package, version 8. Genetics Computer Group, Madison, Wisconsin
Gillis M, Vandamme P, De Vos P, Swings J, Kersters K. (2001). Polyphasic Taxonomy. In: Boone DR, Castenholz RW, Garrity GM (eds) Bergey’s Manual of Systematic Bacteriology, 2ed edn. Vol. 1. Springer, Pringer-Verlag New York Berlin Heidelberg, pp43–48
Graham PH, Sadowsky MJ, Keyser HH, Barnet YM, Bradley RS, Cooper JE, De Ley J, Jarvis BDW, Roslycky EB, Strijdom BW, Young JPW (1991) Proposed minimal standards for the description of new genera and species of root- and stem-nodulating bacteria. Int J Syst Bacteriol 41:582–587
Guo XW, Zhang XX, Zhang ZM, Li FD (1999) Characterization of Astragalus sinicus rhizobia by restriction fragment length polymorphism analysis of chromosomal and nodulation genes regions. Curr Microbiol 39:358–0364
Hurek T, Wagner B, Reinhold-Hurek B (1997) Identification of N2-fixing plant- and fungus-associated Azoarcus species by PCR-based genomic fingerprints Appl Environ Microbiol 63:4331–4339
Hynes MF, McGregor NF (1990) Two plasmids other than the nodulation plasmid are necessary for formation of nitrogen-fixing nodules by Rhizobium leguminosarum. Mol Microbiol 4:567–574
Jarvis BDW (1983) Genetic diversity of Rhizobium strains which nodulate Leucaena leucocephala. Curr Microbiol 8:153–158
Jarvis BDW, Pankhurst CE, Patel JJ (1982) Rhizobium loti, a new species of legume root nodule bacteria. Int J Syst Bacteriol 32:378–380
Martínez-Romero E, Caballero-Mellado J (1996) Rhizobium phylogenies and bacterial genetic diversity. Crit Rev Plant Sci 15:113–140
Martínez-Romero E, Segovia L, Mercante FM, Franco AA, Graham P, Pardo MA (1991) Rhizobium tropici, a novel species nodulating Phaseolus vulgaris L. beans and Leucaena sp. trees. Int J Syst Bacteriol 41:417–426
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 76:5269–5273
Nour SM, Fernandez MP, Normand P, Cleyet-Marel J.-C (1994) Rhizobium ciceri sp. nov., consisting of strains that nodulate chickpeas (Cicer arietinum L.). Int J Syst Bacteriol 4:511–522
Nour SM, Cleyet-Marel J-C, Normand P, Fernandez MP (1995) Genomic heterogeneity of strains nodulating chickpeas (Cicer arietinum L.) and description of Rhizobium mediterraneum sp. nov. Int J Syst Bacteriol 45:640–648
Page RD (1996) Tree View: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Peoples MB, Ladha JK, Herridge DF (1995) Enhancing legume N2 fixation through plant and soil management. Plant and Soil 174:83–101
Piñero D, Martínez E, Selander RK (1988) Genetic diversity and relationshipsamong isolates of Rhizobium leguminosarum biovar phaseoli. Appl Environ Microbiol 54:2825–2832
Rogel MA, Hernández-Lucas I, Kuykendall LD, Balkwill DL, Martínez-Romero E. (2001). Nitrogen-fixing nodules with Ensifer adhaerens harboringg Rhizobium tropici symbiotic plasmids. Appl Environ Microbiol 67:3264–3268
Sanginga N, Vanlauwe B, Danso SKA (1995) Management of biological N2 fixation in alley cropping system: estimation and contribution to N balance. Plant and Soil 174:119–141
Segovia L, Young JPW, Martínez-Romero E (1993) Reclassification of American Rhizobium leguminosarum biovar phaseoli type I strains as Rhizobium etli sp. nov. Int J Syst Bacteriol. 43:374–377
Selander RK, Caugant DA, Ochman H, Musser JM, Gilmour MN, Whittam TS (1986) Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol 51:873–884
Sneath PHA, Sokal RR (1973) Numerical Taxonomy. Freeman, San Francisco
Souza V, Nguyen TT, Hudson RR, Piñero D, Lenski RE (1992) Hierarchical analysis of linkage disequilibrium in Rhizobium populations: evidence for sex? Proc Natl Acad Sci USA 89:8389–8393
Sullivan JT, Patrick HN, Lowther WL, Scott DB, Ronson CW (1995) Nodulating strains of Rhizobium loti arise through chromosomal symbiotic gene transfer in the environment. Proc Natl Acad Sci U S A. 92:8985–8989
Tan ZY, Wang ET, Peng GX, Zhu ME, Martínez-Romero E, Chen WX (1999) Characterization of bacteria isolated from wild legumes in the Northwestern regions of China Int J Syst Bacteriol 49:1457–1469
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weigh matrix choice. Nucleic Acids Res 22:4673–4680
Trinick MJ (1980) Relationships among the fast-growing rhizobia of Lablab pyrpureus, Leucaena leucocephala, Mimosa spp. Acacia farnesiana ana Sesbania grandifora and their affinities with other Rhizobium groups. J Appl Bacteriol 49:39–53
Velázquez E, Igual JM, Willems A, Fernández MP, Muñoz E, Mateos PF, Abril A, Toro N, Normand P, Cervantes E, Gilli M, Martínez-Molina E (2001) Mesorhizobium chacoense sp. nov., a novel species that nodulates Prosopis alba in the Chaco Arido region (Argentina). Int J Syst Evol Microbiol 51:1011–1021
Wang ET, Martínez-Romero E (2000) Sesbania herbacea-Rhizobium huautlense nodulation in flooded soils and comparative characterization of S. herbacea-nodulating rhizobia in different environments. Microbiol Ecol 40:25–32
Wang ET, van Berkum P, Beyene D, Sui XH, Dorado O, Chen WX, Martínez-Romero E (1998) Rhizobium huautlense sp. nov., a symbiont of Sesbania herbacea that has a close phylogenetic relationship with Rhizobium galegae. Int J Syst Bacteriol 48:687–699
Wang ET, van Berkum P, Sui XH, Beyene D, Chen WX, Martínez-Romero E (1999a) Diversity of rhizobia associated with Amorpha fruticosa isolated from Chinese soils and description of Mesorhizobium amorphae sp. nov. Int J Syst Bacteriol 49:51–65
Wang ET, Martínez-Romero J, Martínez-Romero E (1999b) Genetic diversity of rhizobia nodulating Leucaena leucocephala in Mexican soils. Mol Ecol 8:711–724
Ward DM (1998) A natural species concept for prokaryotes. Curr Opin Microbiol 1:271–277
Wayne LG, Brenner DJ, Colwell RR, Grimont PAD, Kandler O, Krichevsky MI, Moore LH, Moore WEC, Murray RGE, Stackebrandt E, Starr MP, Trüper HG (1987) Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int J Syst Bacteriol. 37:463–464
Yan AM, Wang ET, Kan FL, Tan ZY, Sui XH, Reinhold-Hurek B, Chen WX (2000) Sinorhizobium meliloti associated with Medicago sativa and Melilotus spp. in arid saline soils in Xinjiang, China. Int J Syst Evol Microbiolo 50:1887–1891
Yelton MM, Yang SS, Edie SA, Lim ST (1983) Characterization of an effective salt-tolerant, fast-growing strain of Rhizobium japonicum. J Gen Microbiol 129:1537–1547
Young JPW (1985) Rhizobium population genetics: enzyme polymorphism in isolates from peas, clover, beans and Lucerne grown at the same site. J Gen Microbiol 131:2399–2408
Young JPW, Wexler M (1988) Sym plasmid and chromosomal genotypes are correlated in field populations of Rhizobium leguminosarum. J Gen Microbiol 134:2731–2739
Young JPW, Demetriou L, Apte RG (1987) Rhizobium population genetics: enzyme polymorphosm in Rhizobium leguminosarum from plant and soil in a pea crop. Appl Environ Microbiol 53:397–402
Acknowledgements
We thank M. Antonio Rogel and Julio Martínez-Romero for their technical support. Partial financial support was from grant IN202097 of DGAPA, UNAM, Mexico, from grant 34123-N of CONACyT, Mexico, and from grant 2001CB108905 supported by the National Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, E.T., Kan, F.L., Tan, Z.Y. et al. Diverse Mesorhizobium plurifarium populations native to Mexican soils. Arch Microbiol 180, 444–454 (2003). https://doi.org/10.1007/s00203-003-0610-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-003-0610-z