Abstract
Summary
The present systematic review aimed to evaluate bone mineral density (BMD) change and complication rates of elcatonin on treating postmenopausal osteoporosis. The result confirmed efficacy of elcatonin and safety in combination therapies of elcatonin (C-E).
Introduction
Postmenopausal osteoporosis is an important issue in global aging trends. One treatment of osteoporosis is elcatonin, a kind of calcitonin. However, it has been challenged for long time because of safety. Many trials investigated on this topic, but they were designed differently. Those designs can be categorized in monotherapy of elcatonin (M-E) and C-E. Unfortunately, no synthesized evidence dealt this topic.
Methods
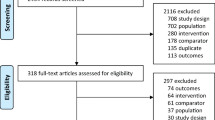
This study systematically identified target trials from six important databases and only included randomized controlled trial for synthesis. Two investigators assessed quality of eligible trials using the Cochrane Risk of Bias Tool, and they independently extracted data. Network meta-analysis performed Peto odds ratio (POR, used for dealing with zero cell) or weighted mean difference (WMD, for continuous data) with 95% confidence intervals (CI) and consistency H.
Results
Sixteen trials recruiting 2754 women with postmenopausal osteoporosis were included in our study. Elcatonin therapies and non-elcatonin medications had comparable fracture rates and bone mineral density change. Yet, C-E (WMD, − 18.93; 95% CI, − 23.97 to − 13.89) and M-E (WMD, − 13.72; 95% CI, − 19.51 to − 7.94) had significantly lower pain score than non-elcatonin medications. However, M-E (POR = 8.413, 95% CI, 2.031 to 34.859) and non-elcatonin medication (Peto OR, 7.450; 95% CI, 1.479 to 37.530) had significantly higher complication rates than placebo. No evidence detected inconsistency and small study effect in this network model.
Conclusions
Based on current evidence, C-E may be considered for treating postmenopausal osteoporosis because it benefits on pain relief and complications. Moreover, it shows comparable fracture rate and bone mineral density change as compared with anti-osteoporosis and calcium supplements. Nevertheless, further trials are needed to investigate formula and dosages of elcatonin.



Similar content being viewed by others
Abbreviations
- BMD:
-
Bone mineral density
- CI:
-
Confidence interval
- C-E:
-
Combination therapy of elcatonin
- LFK:
-
Luis Furuya-Kanamori
- M-E:
-
Monotherapy of elcatonin
- POR:
-
Peto odds ratio
- RCT:
-
Randomized clinical trial
References
NIH Consensus Development Panel on Osteoporosis Prevention D, Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795
Hongo M, Miyakoshi N, Kasukawa Y, Ishikawa Y, Shimada Y (2015) Additive effect of elcatonin to risedronate for chronic back pain and quality of life in postmenopausal women with osteoporosis: a randomized controlled trial. J Bone Miner Metab 33:432–439
Tanaka S, Yoshida A, Kono S, Oguma T, Hasegawa K, Ito M (2016) Effectiveness of elcatonin for alleviating pain and inhibiting bone resorption in patients with osteoporotic vertebral fractures. J Bone Miner Metab
Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, Oden A, Zethraeus N, Pfleger B, Khaltaev N (2005) Assessment of fracture risk. Osteoporos Int 16:581–589
Yoshimura N, Muraki S, Oka H, Kawaguchi H, Nakamura K, Akune T (2010) Cohort profile: Research on Osteoarthritis/Osteoporosis Against Disability study. Int J Epidemiol 39:988–995
Morikawa T, Munekata E, Sakakibara S, Noda T, Otani M (1976) Synthesis of eel-calcitonin and (asu1,7)-eel-calcitonin: contribution of the disulfide bond to the hormonal activity. Experientia 32:1104–1106
Fujita T, Fujii Y, Goto B, Miyauchi A, Takagi Y (1997) A three-year comparative trial in osteoporosis treatment: effect of combined alfacalcidol and elcatonin. J Bone Miner Metab 15:223–226
Knopp JA, Diner BM, Blitz M, Lyritis GP, Rowe BH (2005) Calcitonin for treating acute pain of osteoporotic vertebral compression fractures: a systematic review of randomized, controlled trials. Osteoporos Int 16:1281–1290
Yoh K, Tanaka K, Ishikawa A, Ishibashi T, Uchino Y, Sato Y, Tobinaga M, Hasegawa N, Kamae S, Yoshizawa M (2005) Health-related quality of life (HRQOL) in Japanese osteoporotic patients and its improvement by elcatonin treatment. J Bone Miner Metab 23:167–173
Barendregt JJ, Doi SA (2016) MetaXL user guide. Version 4:2011–2016
Higgins JP, Green S (2008) Cochrane handbook for systematic reviews of interventions
Astengo F, Della Monica A, Fontana L, Fuliano P, Bigolari M (1994) Efficacy and safety of two elcatonin regimens in the treatment of osteoporosis. Curr Ther Res Clin Exp 55:1518–1526
Consoli V, Alfieri P, Giuntini C, Manca M, Avaldi F, Soncini R (1991) A double-blind placebo-controlled trial of the efficacy and tolerability of intranasal elcatonin administered to patients suffering from senile and postmenopausal osteoporosis. Curr Ther Res Clin Exp 50:369–378
Endo N, Fujino K, Doi T, Akai M, Hoshino Y, Nakano T, Iwaya T (2017) Effect of elcatonin versus nonsteroidal anti-inflammatory medications for acute back pain in patients with osteoporotic vertebral fracture: a multiclinic randomized controlled trial. J Bone Miner Metab 35:375–384
Fujita T, Fujii Y, Miyauchi A, Takagi Y (1999) Comparison of antiresorptive activities of ipriflavone, an isoflavone derivative, and elcatonin, an eel carbocalcitonin. J Bone Miner Metab 17:289–295
Iwamoto J, Makita K, Sato Y, Takeda T, Matsumoto H (2011) Alendronate is more effective than elcatonin in improving pain and quality of life in postmenopausal women with osteoporosis. Osteoporos Int 22:2735–2742
Li Y, Xuan M, Wang B et al (2013) Comparison of parathyroid hormone (1-34) and elcatonin in postmenopausal women with osteoporosis: an 18-month randomized, multicenter controlled trial in China. Chin Med J 126:457–463
Lobianco R, Merola B, Lupoli G, Cocca A, Guarino M, Pia M, Guerriero S, Lombardi G (1992) Randomized comparative study using carbocalcitonin i.m. vs carbocalcitonin nasal spray vs ipriflavone x os in the treatment of post-menopausal osteoporosis. Minerva Endocrinol 17:79–84
Lozano-Tonkin C, Gracia Canales A, Jareño J, Mercadal J (2000) Long-term double-blind study of the efficacy of intermitent nasal elcatonin in recent postmenopausal women. An Med Interna 17:399–405
Meschia M, Brincat M, Barbacini P, Carena Maini M, Marri R, Crosignani PG (1992) Effect of hormone replacement therapy and calcitonin on bone mass in postmenopausal women. Eur J Obstet Gynecol Reprod Biol 47:53–57
Pérez-Jaraiz MD, Revilla M, Alvarez De Los Heros JI, Villa LF, Rico H (1996) Prophylaxis of osteoporosis with calcium, estrogens and/or eelcatonin: comparative longitudinal study of bone mass. Maturitas 23:327–332
Sugimoto T, Shiraki M, Nakano T, et al (2018) A randomized, double-blind, placebo-controlled study of once weekly elcatonin in primary postmenopausal osteoporosis. Curr Med Res Opin 1–8
Tanaka S, Yoshida A, Kono S, Ito M (2017) Effectiveness of monotherapy and combined therapy with calcitonin and minodronic acid hydrate, a bisphosphonate, for early treatment in patients with new vertebral fractures: an open-label, randomized, parallel-group study. J Orthop Sci 22:536–541
Yang Y, Zhang X-J, Zhu X-J, Zhang L, Bao M-J, Xian Y, Wu J-C, Liu L-M, Li P-Q (2015) Comparison between recombinant human parathyroid hormone (1–34) and elcatonin in treatment of primary osteoporosis. Asian Pac J Trop Med 8:79–84
Zhang L, Li L, Yang M, Xu K, Boden G, Yang G (2013) The rhPTH treatment elevates plasma secreted protein acidic and rich in cysteine levels in patients with osteoporosis. Osteoporos Int 24:1107–1112
Zhang XZ, Song LG, Wang B et al (2010) A randomized, multicenter, active-controlled trial to compare the efficacy of recombinant human parathyroid hormone (1-34) with that of elcatonin in postmenopausal women with osteoporosis in China. Zhonghua Nei Ke Za Zhi 49:662–666
Zhang XZ, Wang B, Yang J et al (2009) A randomized, multicenter controlled trial to compare the efficacy of recombinant human parathyroid hormone (1-34) with elcatonin in postmenopausal women with osteoporosis in China. Chin Med J 122:2933–2938
Ivaska KK, Gerdhem P, Akesson K, Garnero P, Obrant KJ (2007) Effect of fracture on bone turnover markers: a longitudinal study comparing marker levels before and after injury in 113 elderly women. J Bone Miner Res Off J Am Soc Bone Miner Res 22:1155–1164
Silverman SL, Azria M (2002) The analgesic role of calcitonin following osteoporotic fracture. Osteoporos Int 13:858–867
Shibata K, Takeda M, Ito A, Takeda M, Sagai H (1998) Ovariectomy-induced hyperalgesia and antinociceptive effect of elcatonin, a synthetic eel calcitonin. Pharmacol Biochem Behav 60:371–376
Committee TEMAs (2012) European Medicines Agency recommends limiting longterm use of calcitonin medicines. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2012/07/WC500130122.pdf. Accessed 29 Aug 2018
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 573 kb)
Rights and permissions
About this article
Cite this article
Chen, WC., Lin, EY. & Kang, YN. Efficacy and safety of elcatonin in postmenopausal women with osteoporosis: a systematic review with network meta-analysis of randomized clinical trials. Osteoporos Int 30, 1723–1732 (2019). https://doi.org/10.1007/s00198-019-04997-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-019-04997-6