Abstract
Summary
Two alendronate-EP4 agonist (ALN-EP4a) conjugate drugs, C1 and C2, which differ in structure by a short linker molecule, were evaluated in ovariectomized (OVX) rats for their anabolic effects. We showed that C1 led to significant anabolic effects on cortical and trabecular bone while anabolic effects associated with C2 were minimal.
Introduction
EP4as were covalently linked to ALN to create ALN-EP4a conjugate anabolic bone drugs, C1 and C2, which differ in structure by a short linker molecule in C1. When administered systemically, C1 and C2 are delivered to bone through targeted binding of ALN, where local hydrolytic enzymes liberate EP4a from ALN to exert anabolic effects. Here, we compare effects of C1 to C2 in a curative in vivo study.
Methods
Three-month-old female Sprague Dawley rats were OVX or sham operated and allowed to lose bone for 3 months. Animals were then treated via tail vein injections for 3 months and sacrificed. Treatment groups were as follows: C1L (5 mg/kg biweekly), C1H (5 mg/kg weekly), C2L (15 mg/kg monthly), C2H (15 mg/kg biweekly), OVX and sham control (phosphate-buffered saline (PBS) biweekly), and ALN/EP4a-unconjugated mixture (0.75 mg/kg each biweekly).
Results
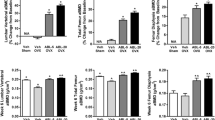
MicroCT analysis showed that C1H treatment significantly increased vertebral bone mineral density (vBMD) and trabecular bone volume versus OVX controls while C2 treatments did not. Biomechanical testing showed that C1H treatment but not C2 treatments led to significant improvement in the load bearing abilities of the vertebrae compared to OVX controls. C1 stimulated endocortical bone formation and increased load bearing in femurs, while C2 did not.
Conclusions
We showed that C1 led to significant anabolic effects on cortical and trabecular bone while anabolic effects associated with C2 were minimal. These results led us to hypothesize a mode of action by which presence of a linker is crucial in facilitating the anabolic effects of EP4a when dosed as a prodrug with ALN.




Similar content being viewed by others
References
Russell RG, Watts NB, Ebetino FH, Rogers MJ (2008) Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 19(6):733–759. doi:10.1007/s00198-007-0540-8
Green JR (2001) Chemical and biological prerequisites for novel bisphosphonate molecules: results of comparative preclinical studies. Semin Oncol 28(2 Suppl 6):4–10
Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 280(24):2077–2082
Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, Nevitt MC, Suryawanshi S, Cummings SR (2000) Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab 85(11):4118–4124. doi:10.1210/jcem.85.11.6953
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348(9041):1535–1541
Allen MR, Iwata K, Phipps R, Burr DB (2006) Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone 39(4):872–879. doi:10.1016/j.bone.2006.04.028
Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY (2005) Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 90(3):1294–1301. doi:10.1210/jc.2004-0952
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH (2001) Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344(19):1434–1441. doi:10.1056/nejm200105103441904
Tsuchie H, Miyakoshi N, Kasukawa Y, Aonuma H, Shimada Y (2013) Intermittent administration of human parathyroid hormone before osteosynthesis stimulates cancellous bone union in ovariectomized rats. Tohoku J Exp Med 229(1):19–28
Meng XW, Liang XG, Birchman R, Wu DD, Dempster DW, Lindsay R, Shen V (1996) Temporal expression of the anabolic action of PTH in cancellous bone of ovariectomized rats. J Bone Miner Res 11(4):421–429. doi:10.1002/jbmr.5650110402
Tashjian AH Jr, Gagel RF (2006) Teriparatide [human PTH(1-34)]: 2.5 years of experience on the use and safety of the drug for the treatment of osteoporosis. J Bone Miner Res 21(3):354–365. doi:10.1359/jbmr.051023
Jolette J, Wilker CE, Smith SY, Doyle N, Hardisty JF, Metcalfe AJ, Marriott TB, Fox J, Wells DS (2006) Defining a noncarcinogenic dose of recombinant human parathyroid hormone 1-84 in a 2-year study in Fischer 344 rats. Toxicol Pathol 34(7):929–940. doi:10.1080/01926230601072301
Vahle JL, Long GG, Sandusky G, Westmore M, Ma YL, Sato M (2004) Bone neoplasms in F344 rats given teriparatide [rhPTH(1-34)] are dependent on duration of treatment and dose. Toxicol Pathol 32(4):426–438. doi:10.1080/01926230490462138
Collins DA, Chambers TJ (1992) Prostaglandin E2 promotes osteoclast formation in murine hematopoietic cultures through an action on hematopoietic cells. J Bone Miner Res 7(5):555–561. doi:10.1002/jbmr.5650070512
Li X, Okada Y, Pilbeam CC, Lorenzo JA, Kennedy CR, Breyer RM, Raisz LG (2000) Knockout of the murine prostaglandin EP2 receptor impairs osteoclastogenesis in vitro. Endocrinology 141(6):2054–2061. doi:10.1210/endo.141.6.7518
Kaneki H, Takasugi I, Fujieda M, Kiriu M, Mizuochi S, Ide H (1999) Prostaglandin E2 stimulates the formation of mineralized bone nodules by a cAMP-independent mechanism in the culture of adult rat calvarial osteoblasts. J Cell Biochem 73(1):36–48
Scutt A, Bertram P (1995) Bone marrow cells are targets for the anabolic actions of prostaglandin E2 on bone: induction of a transition from nonadherent to adherent osteoblast precursors. J Bone Miner Res 10(3):474–487. doi:10.1002/jbmr.5650100320
Ke HZ, Jee WS, Ito H, Setterberg RB, Li M, Lin BY, Liang XG, Ma YF (1993) Greater bone formation induction occurred in aged than young cancellous bone sites. Bone 14(3):481–485
Ke HZ, Jee WS, Zeng QQ, Li M, Lin BY (1993) Prostaglandin E2 increased rat cortical bone mass when administered immediately following ovariectomy. Bone Miner 21(3):189–201
Ke HZ, Li M, Jee WS (1992) Prostaglandin E2 prevents ovariectomy-induced cancellous bone loss in rats. Bone Miner 19(1):45–62
Suponitzky I, Weinreb M (1998) Differential effects of systemic prostaglandin E2 on bone mass in rat long bones and calvariae. J Endocrinol 156(1):51–57
Jee WS, Ma YF (1997) The in vivo anabolic actions of prostaglandins in bone. Bone 21(4):297–304
Tian XY, Zhang Q, Zhao R, Setterberg RB, Zeng QQ, Iturria SJ, Ma YF, Jee WS (2008) Continuous PGE2 leads to net bone loss while intermittent PGE2 leads to net bone gain in lumbar vertebral bodies of adult female rats. Bone 42(5):914–920. doi:10.1016/j.bone.2007.12.228
Lauritzen DB, Balena R, Shea M, Seedor JG, Markatos A, Le HM, Toolan BC, Myers ER, Rodan GA, Hayes WC (1993) Effects of combined prostaglandin and alendronate treatment on the histomorphometry and biomechanical properties of bone in ovariectomized rats. J Bone Miner Res 8(7):871–879. doi:10.1002/jbmr.5650080713
Miyaura C, Inada M, Suzawa T, Sugimoto Y, Ushikubi F, Ichikawa A, Narumiya S, Suda T (2000) Impaired bone resorption to prostaglandin E2 in prostaglandin E receptor EP4-knockout mice. J Biol Chem 275(26):19819–19823. doi:10.1074/jbc.M002079200
Yoshida K, Oida H, Kobayashi T, Maruyama T, Tanaka M, Katayama T, Yamaguchi K, Segi E, Tsuboyama T, Matsushita M, Ito K, Ito Y, Sugimoto Y, Ushikubi F, Ohuchida S, Kondo K, Nakamura T, Narumiya S (2002) Stimulation of bone formation and prevention of bone loss by prostaglandin E EP4 receptor activation. Proc Natl Acad Sci U S A 99(7):4580–4585. doi:10.1073/pnas.062053399
Ke HZ, Crawford DT, Qi H, Simmons HA, Owen TA, Paralkar VM, Li M, Lu B, Grasser WA, Cameron KO, Lefker BA, DaSilva-Jardine P, Scott DO, Zhang Q, Tian XY, Jee WS, Brown TA, Thompson DD (2006) A nonprostanoid EP4 receptor selective prostaglandin E2 agonist restores bone mass and strength in aged, ovariectomized rats. J Bone Miner Res 21(4):565–575. doi:10.1359/jbmr.051110
Arns S, Gibe R, Moreau A, Monzur Morshed M, Young RN (2012) Design and synthesis of novel bone-targeting dual-action pro-drugs for the treatment and reversal of osteoporosis. Bioorg Med Chem 20(6):2131–2140. doi:10.1016/j.bmc.2012.01.024
Papadopoulos G, Boskou D (1991) Antioxidant effect of natural phenols on olive oil. J Am Oil Chem Soc 68(9):669–671. doi:10.1007/BF02662292
Billot X, Chateauneuf A, Chauret N, Denis D, Greig G, Mathieu MC, Metters KM, Slipetz DM, Young RN (2003) Discovery of a potent and selective agonist of the prostaglandin EP4 receptor. Bioorg Med Chem Lett 13(6):1129–1132
Liu CC, Hu S, Chen G, Georgiou J, Arns S, Kumar NS, Young RN, Grynpas MD (2014) Novel EP4 receptor agonist-bisphosphonate conjugate drug (C1) promotes bone formation and improves vertebral mechanical properties in the ovariectomized rat model of postmenopausal bone loss. J Bone Miner Res. doi:10.1002/jbmr.2382
Chen G, Arns S, Young RN (2015) Determination of the rat in vivo pharmacokinetic profile of a bone-targeting dual-action pro-drug for treatment of osteoporosis. Bioconjug Chem. doi:10.1021/acs.bioconjchem.5b00160
Kalu DN (1991) The ovariectomized rat model of postmenopausal bone loss. Bone Miner 15(3):175–191
Wronski TJ, Cintron M, Dann LM (1988) Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcif Tissue Int 43(3):179–183
Gil L, Han Y, Opas EE, Rodan GA, Ruel R, Seedor JG, Tyler PC, Young RN (1999) Prostaglandin E2-bisphosphonate conjugates: potential agents for treatment of osteoporosis. Bioorg Med Chem 7(5):901–919
Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28(1):2–17. doi:10.1002/jbmr.1805
Jee WS, Mori S, Li XJ, Chan S (1990) Prostaglandin E2 enhances cortical bone mass and activates intracortical bone remodeling in intact and ovariectomized female rats. Bone 11(4):253–266
Li M, Thompson DD, Paralkar VM (2007) Prostaglandin E(2) receptors in bone formation. Int Orthop 31(6):767–772. doi:10.1007/s00264-007-0406-x
Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB (2000) Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res 15(4):613–620. doi:10.1359/jbmr.2000.15.4.613
Flanagan AM, Chambers TJ (1992) Stimulation of bone nodule formation in vitro by prostaglandins E1 and E2. Endocrinology 130(1):443–448. doi:10.1210/endo.130.1.1309342
Blackwell KA, Raisz LG, Pilbeam CC (2010) Prostaglandins in bone: bad cop, good cop? Trends Endocrinol Metab 21(5):294–301. doi:10.1016/j.tem.2009.12.004
Mano M, Arakawa T, Mano H, Nakagawa M, Kaneda T, Kaneko H, Yamada T, Miyata K, Kiyomura H, Kumegawa M, Hakeda Y (2000) Prostaglandin E2 directly inhibits bone-resorbing activity of isolated mature osteoclasts mainly through the EP4 receptor. Calcif Tissue Int 67(1):85–92
Sakuma Y, Tanaka K, Suda M, Yasoda A, Natsui K, Tanaka I, Ushikubi F, Narumiya S, Segi E, Sugimoto Y, Ichikawa A, Nakao K (2000) Crucial involvement of the EP4 subtype of prostaglandin E receptor in osteoclast formation by proinflammatory cytokines and lipopolysaccharide. J Bone Miner Res 15(2):218–227. doi:10.1359/jbmr.2000.15.2.218
Tomita M, Li X, Okada Y, Woodiel FN, Young RN, Pilbeam CC, Raisz LG (2002) Effects of selective prostaglandin EP4 receptor antagonist on osteoclast formation and bone resorption in vitro. Bone 30(1):159–163
Rodan GA, Seedor JG, Balena R (1993) Preclinical pharmacology of alendronate. Osteoporos Int 3(Suppl 3):S7–12
Acknowledgments
We thank the following individuals for their assistance in this study: Adeline Ng, Ariana Dela Cruz, Elise Post, and Richard Cheung for general experimental techniques and technical assistance; Kate Banks for animal care; Douglas Holmyard for BSE; and Richard Renlund for animal pathology. This work was supported by a Collaborative Health Research Grant from the Natural Sciences and Engineering Research Council of Canada and the Canadian Institutes of Health Research (Institute of Musculoskeletal Health and Arthritis), awarded to RNY and MDG, Grant number CHRPJ 351731-08. SH was supported by a research fellowship from the University of Toronto.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, S., Liu, C.C., Chen, G. et al. In vivo effects of two novel ALN-EP4a conjugate drugs on bone in the ovariectomized rat model for reversing postmenopausal bone loss. Osteoporos Int 27, 797–808 (2016). https://doi.org/10.1007/s00198-015-3284-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3284-x