Abstract
Summary
A 12-month extension phase of DIRECT in Japanese subjects with osteoporosis showed that total 3 years of denosumab treatment in Japanese postmenopausal women and men with osteoporosis was associated with low fracture rates, persistent bone turnover marker (BTM) reductions, continuous bone mineral density (BMD) increases, and a favorable overall benefit/risk profile.
Introduction
The DIRECT trial demonstrated that 2 years of treatment with denosumab 60 mg subcutaneously every 6 months significantly reduced the incidence of vertebral fracture compared to placebo in Japanese postmenopausal women and men with osteoporosis. The purpose of this study is to evaluate the efficacy and safety of denosumab treatment for up to 3 years.
Methods
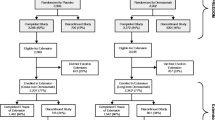
This study includes a 2-year randomized, double-blind, placebo-controlled phase and a 1-year open-label extension phase in which all subjects received denosumab. The data correspond to 3 years of denosumab treatment in subjects who received denosumab (long-term group) and 1 year of denosumab treatment in subjects who received placebo (cross-over group) in the double-blind phase.
Results
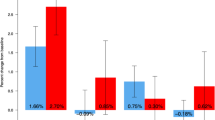
Eight hundred and ten subjects who completed the double-blind phase enrolled into the extension phase, and 775 subjects completed the study. All subjects received denosumab with daily supplements of calcium and vitamin D. The cumulative 36-month incidences of new or worsening vertebral fractures and new vertebral fractures were 3.8 and 2.5 %, respectively, in the long-term group. In this group, the BMD continued to increase, and the reduction in BTMs was maintained. In the cross-over group, comparable BMD increases and BTMs reductions to those of in their first year of the long-term group were confirmed. Adverse events did not show a notable increase with long-term denosumab administration. One event of osteonecrosis of the jaw occurred in the cross-over group.
Conclusions
Three-year denosumab treatment in Japanese subjects with osteoporosis showed a favorable benefit/risk profile.



Similar content being viewed by others
References
NIH (2001) Consensus conference (2001) osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795
Hochberg MC, Greenspan S, Wasnich RD, Miller P, Thompson DE, Ross PD (2002) Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab 87(4):1586–1592
Ivaska KK, Gerdhem P, Väänänen HK, Akesson K, Obrant KJ (2010) Bone turnover markers and prediction of fracture: a prospective follow-up study of 1040 elderly women for a mean of 9 years. J Bone Miner Res 25(2):393–403
Fuller K, Wong B, Fox S, Choi Y, Chambers TJ (1998) TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med 188(5):997–1001
Lacey DL, Tan HL, Lu J, Kaufman S, Van G, Qiu W, Rattan A, Scully S, Fletcher F, Juan T, Kelley M, Burgess TL, Boyle WJ, Polverino AJ (2000) Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol 157(2):435–448
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93(2):165–176
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A 95(7):3597–3602
Cummings SR, Martin JS, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Christiansen C (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Papapoulos S, Chapurlat R, Libanati C, Brandi ML, Brown JP, Czerwinski E, Krieg M-A, Man Z, Mellstrom D, Radominski SC, Reginster J-Y, Resch H, Ivorra JAR, Roux C, Vittinghoff E, Austin M, Daizadeh N, Bradley MN, Grauer A, Cummings SR, Bone HG (2012) Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension. J Bone Miner Res 27(3):694–701
Bone HG, Chapurlat R, Brandi ML, Brown JP, Czerwinski E, Krieg MA, Mellström D, Radominski SC, Reginster JY, Resch H, Ivorra JA, Roux C, Vittinghoff E, Daizadeh NS, Wang A, Bradley MN, Franchimont N, Geller ML, Wagman RB, Cummings SR, Papapoulos S (2013) The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab 98(11):4483–4492
Papapoulos S, Lippuner K, Roux C, Hall J, Kendler D, Lewiecki EM, Brandi ML, Czerwiński E, Franek E, Lakatos P, Mautalen C, Minisola S, Reginster JY, Jensen S, Daizadeh N, Wang A, Geller M, Wagman RB, Bone HG (2013) Eight Years of Denosumab Treatment in Postmenopausal Women With Osteoporosis: Results From the First Five Years of the FREEDOM Extension [Abstract LB-MO26] American Society for Bone and Mineral Research 2013 Annual Meeting
Nakamura T, Matsumoto T, Sugimoto T, Hosoi T, Miki T, Gorai I, Yoshikawa H, Tanaka Y, Tanaka S, Sone T, Nakano T, Ito M, Matsui S, Yoneda T, Takami H, Watanabe K, Osakabe T, Shiraki M, Fukunaga M (2014) Fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: denosumab fracture intervention randomized placebo controlled trial (DIRECT). J Clin Endocrinol Metab 99(7):2599–2607
Genant HK, Jergas M, Palermo L, Nevitt M, Valentin RS, Black D, Cummings SR (1996) Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. J Bone Miner Res 11(7):984–996
Orimo H, Sugioka Y, Fukunaga M, Muto Y, Hotokebuchi T, Gorai I, Nakamura T, Kushida K, Ikai H, Tanaka T, Oh-hashi Y (1998) Diagnostic criteria of primary osteoporosis. J Bone Miner Metab 16:139–150
Kumagai Y, Hasunuma T, Padhi D (2011) A randomized, double-blind, placebo-controlled, single-dose study to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of denosumab administered subcutaneously to postmenopausal Japanese women. Bone 49(5):1101–1107
Austin M, Yang YC, Vittinghoff E, Adami S, Boonen S, Bauer DC, Bianchi G, Bolognese MA, Christiansen C, Eastell R, Grauer A, Hawkins F, Kendler DL, Oliveri B, McClung MR, Reid IR, Siris ES, Zanchetta J, Zerbini CA, Libanati C, Cummings SR (2012) FREEDOM Trial. Relationship between bone mineral density changes with denosumab treatment and risk reduction for vertebral and nonvertebral fractures. J Bone Miner Res 27(3):687–693
Acknowledgments
The study was sponsored by Daiichi-Sankyo., Tokyo, Japan. The authors acknowledge Amgen for editorial assistance on the manuscript.
Conflicts of interest
T. Sugimoto has received consulting fees from Asahi-Kasei Pharma, and Daiichi-Sankyo. He has received research grants from Eli Lilly Japan, Eisai, MSD, Taisho Toyama Pharmaceutical, Chugai Pharmaceutical, Daiichi-Sankyo, and Pfizer. T. Matsumoto has received consulting fees from Daiichi-Sankyo, Ono Pharmaceutical., Asahi-Kasei Pharma., Chugai Pharmaceutical, Astellas Pharma, and Teijin Pharma. T. Hosoi, T. Miki, and I. Gorai have received consulting fees from Daiichi-Sankyo. H. Yoshikawa has received consulting fees from Ono Pharmaceutical, Asahi-Kasei Pharma, and Eisai. He has also received speaker’s bureau from Asahi-Kasei Pharma, Ono Pharmaceutical, Astellas Pharma, and Eli Lilly Japan. Y. Tanaka has received consulting fees from Mitsubishi-Tanabe Pharma, Otsuka Pharma, Pfizer, Eli Lilly Japan, Astellas Pharma, Janssen Pharma, Astra-Zeneca, Mitsubishi-Tanabe Pharma, Abbott Japan, MSD, Daiichi-Sankyo, GlaxoSmithKline, Actelion Pharma Japan, Chugai Pharmaceutical, Eisai, and Santen Pharma. He has also received research grants from Eisai, Pfizer, Janssen Pharma, Astellas Pharma, Bristol-Myers Squibb, Abbott Japan, and Chugai Pharmaceutical. S. Tanaka has received speaker's bureau from Eli Lilly Japan, Janssen Pharmaceutical, Eisai, Santen Pharmaceutical, Teijin Pharma, Nippon Zoki, Johnson & Johnson, Chugai Pharmaceutical, Daiichi-Sankyo, Ono Pharmaceutical, GSK, Pfizer, Mitsubishi Tanabe Pharma, Hisamitsu Pharmaceutical, Asahi Kasei Pharma, Taisho Toyama Pharmaceutical, Astellas Pharma, and Otsuka Pharmaceutical. He has also received consulting fees from Eisai, Astellas Pharma, Bristol-Myers Squibb, Pfizer, Takeda Pharmaceutical, Asashi Kasei Pharma, Ono Pharmaceutical, Dainippon Sumitomo Pharma and Kaken Pharmaceutical, and research grants from Sanofi Aventis, Daiichi-Sankyo, and MSD. T. Nakano has received consulting fees from Asahi-Kasei Pharma, Teijin Pharma, Daiichi-Sankyo, and Chugai Pharmaceutical. M. Ito has received consulting fees from Asahi-Kasei Pharma, Daiichi-Sankyo, and Chugai Pharmaceutical. M. Fukunaga has received consulting fees from Asahi-Kasei Pharma and Astellas Pharma. T. Sone has received research grants from Daiichi-Sankyo Astellas Pharma, Takeda Pharmaceutical, Taisho Toyama Pharmaceutical, and Pfizer and speaker’s bureau from Daiichi-Sankyo, Chugai Pharmaceutical, MSD, Taisho Toyama Pharmaceutical, Pfizer, and Eli Lilly Japan. T. Yoneda has received consulting fees from Daiichi-Sankyo. S. Matsui has received consulting fees from Chugai Pharmaceutical, Daiichi-Sankyo, Eisai, and Takada Pharmaceutical. H. Takami, K Watanabe, T. Osakabe, and N. Okubo are Daiichi-Sankyo employees and own Daiichi-Sankyo stock. M Shiraki has received consulting fees from Daiichi-Sankyo, Chugai Pharmaceutical, Teijin Pharma, Asahi-Kasei Pharma and MSD. T. Nakamura has received consulting fees from Teijin Pharma, Daiichi-Sankyo, Chugai Pharmaceutical, Asahi-Kasei Pharma, and Amgen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugimoto, T., Matsumoto, T., Hosoi, T. et al. Three-year denosumab treatment in postmenopausal Japanese women and men with osteoporosis: results from a 1-year open-label extension of the Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT). Osteoporos Int 26, 765–774 (2015). https://doi.org/10.1007/s00198-014-2964-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-014-2964-2