Abstract
Summary
Treatment rates of osteoporosis after fracture are very low. Women who suffer a fragility fracture have a greater chance of receiving anti-fracture treatment if they had low bone mineral density (BMD), a fracture at the hip, femur or pelvis, administration of calcium and vitamin D supplements and/or an age ≥60 years.
Introduction
This investigation identifies the predictors of osteoporosis treatment 6 to 8 months following fragility fracture in women >50 years of age.
Methods
In this prospective cohort study, women were recruited 0 to 16 weeks following fracture and classified as having experienced fragility or traumatic fractures (phase 1). Six to 8 months following fracture, women completed a questionnaire on demographic features, clinical characteristics and risk factors for osteoporosis (phase 2). Osteoporosis treatment was defined as initiating anti-fracture therapy (bisphosphonate, raloxifene, nasal calcitonin and teriparatide) after fracture in those previously untreated.
Results
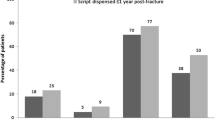
Of the 1,273 women completing phase 1, 1,001 (79%) sustained a fragility fracture, and of these women, 738 were untreated for osteoporosis at phase 1 and completed the phase 2 questionnaire. Significant predictors of treatment included BMD result, fracture site, administration of calcium and vitamin D supplements at the time of fracture and age ≥60 years. All other risk factors for osteoporosis, such as fracture history after the age of 40 years, family history of osteoporosis and comorbidities did not significantly influence the treatment rate.
Conclusions
Physicians largely based their decision to treat on BMD results and not on the clinical event—fragility fracture.



Similar content being viewed by others
References
Cooper C (1997) The crippling consequences of fractures and their impact on quality of life. Am J Med 103:12S–17S discussion 17S–19S
Melton LJ 3rd (2003) Adverse outcomes of osteoporotic fractures in the general population. J Bone Miner Res 18:1139–1141
Randell A, Sambrook PN, Nguyen TV, Lapsley H, Jones G, Kelly PJ, Eisman JA (1995) Direct clinical and welfare costs of osteoporotic fractures in elderly men and women. Osteoporos Int 5:427–432
Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jonsson B (2004) Mortality after osteoporotic fractures. Osteoporos Int 15:38–42
Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jonsson B (2004) Fracture risk following an osteoporotic fracture. Osteoporos Int 15:175–179
Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, Eisman J, Fujiwara S, Garnero P, Kroger H (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35:375–382
Doherty DA, Sanders KM, Kotowicz MA, Prince RL (2001) Lifetime and five-year age-specific risks of first and subsequent osteoporotic fractures in postmenopausal women. Osteoporos Int 12:16–23
van Staa TP, Leufkens HG, Cooper C (2002) Does a fracture at one site predict later fractures at other sites? A British cohort study. Osteoporos Int 13:624–629
Klotzbuecher CM, Ross PD, Landsman PB, Abbott TAI, Berger M (2000) Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 15:721–739
Mallmin H, Ljunghall S, Persson I, Naessen T, Krusemo UB, Bergstrom R (1993) Fracture of the distal forearm as a forecaster of subsequent hip fracture: a population-based cohort study with 24 years of follow-up. Calcif Tissue Int 52:269–272
Robinson CM, Royds M, Abraham A, McQueen MM, Court-Brown CM, Christie J (2002) Refractures in patients at least forty-five years old. a prospective analysis of twenty-two thousand and sixty patients. J Bone Joint Surg Am 84-A:1528–1533
Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, Croix AZL (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures. JAMA 280:2077–2082
Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, Nevitt MC, Suryawanshi S, Cummings SR (2000) Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab 85:4118–4124
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA 282:1344–1352
Reginster J-Y, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, Lund B, Ethgen D, Pack S, Roumagnac I, Eastell R (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int 11:83–91
Chesnut CH, Silverman S, Andriano K, Genant H, Gimona A, Harris S, Kiel D, Boff ML, Maricic M, Miller P, Moniz C, Peacock M, Richardson P, Watts N, Baylink D, Group ftPS (2000) A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. Am J Med 109:267–276
Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Gluer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR, Investigators ftMOoREM (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene. Results from a 3-year randomized clinical trial. JAMA 282:637–645
McClung MR, Geusens P, Miller PD, Zippel H, Bensen W, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier P, Wasnich R, Greenwald M, Kaufman J, Chesnut C, Reginster J, Group ftHIPHS (2001) Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med 344:333–340
Bessette L, Ste-Marie LG, Jean S, Davison KS, Beaulieu M, Baranci M, Bessant J, Brown JP (2007) The care gap in diagnosis and treatment of women with a fragility fracture. Osteoporos Int 19:79–86
Elliot-Gibson V, Bogoch ER, Jamal SA, Beaton DE (2004) Practice patterns in the diagnosis and treatment of osteoporosis after a fragility fracture: a systematic review. Osteoporos Int 15:767–778
Giangregorio L, Papaioannou A, Cranney A, Zytaruk N, Adachi JD (2006) Fragility fractures and the osteoporosis care gap: an international phenomenon. Semin Arthritis Rheum 35:293–305
Levasseur R, Sabatier JP, Guilcher C, Guaydier-Souquieres G, Costentin-Pignol V, Jean-Jacques PY, Hulet C, Vielpeau C, Marcelli C (2007) Medical management of patients over 50 years admitted to orthopedic surgery for low-energy fracture. Joint Bone Spine 74:160–165
Papaioannou A, Giangregorio L, Kvern B, Boulos P, Ioannidis G, Adachi JD (2004) The osteoporosis care gap in Canada. BMC Musculoskelet Disord 5:11
Vik SA, Maxwell CJ, Hanley DA (2005) Treatment of osteoporosis in an older home care population. BMC Musculoskelet Disord 6:7
Bessette L, Ste-Marie LG, Jean S, Davison KS, Beaulieu M, Baranci M, Bessant J, Brown JP (2007) Recognizing osteoporosis and its consequences in Quebec (ROCQ): Background, rationale, and methods of an anti-fracture patient health-management programme. Contemp Clin Trials 29:194–210
Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A (2001) The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 12:417–427
Ingle BM, Eastell R (2002) Site-specific bone measurements in patients with ankle fracture. Osteoporos Int 13:342–347
Jacobsen D, Sargent J, Atkinson EJ, O'Fallon WM, Melton LJI (1999) Contribution of weather to the seasonality of distal forearm fractures : a population-based study in Rochester, Minnesota. Osteoporos Int 9:254–259
Kannus P, Palvanen M, Niemi S, Parkkari J, Jarvinen M (2000) Epidemiology of osteporotic pelvic fractures in elderly people in Finland : sharp increase in 1970–1997 and alarming projections for the new millenium. Osteoporos Int 11:443–448
Khan SA, de Geus C, Holroyd B, Russell AS (2001) Osteoporosis follow-up after wrist fractures following minor trauma. Arch Intern Med 161:1309–1312
Tromp AM, Smit JH, Deeg DJH, Bouter LM, Lips P (1998) Predictors for falls and fractures in longitudinal aging study Amsterdam. J Bone Miner Res 13:1932–1939
Kreiger N, Tenenhouse A, Joseph L, Mackenzie T, Poliquin S, Brown JP, Prior JC, Rittmaster RS (1999) The Canadian Multicentre Osteoporosis Study (CaMos): background, rationale, methods. Can J Aging 18:376–387
Nguyen ND, Frost SA, Center JR, Eisman JA, Nguyen TV (2007) Development of a nomogram for individualizing hip fracture risk in men and women. Osteoporos Int 18:1109–1117
Richards JB, Leslie WD, Joseph L, Siminoski K, Hanley DA, Adachi JD, Brown JP, Morin S, Papaioannou A, Josse RG, Prior JC, Davison KS, Tenenhouse A, Goltzman D (2007) Changes to osteoporosis prevalence according to method of risk assessment. J Bone Miner Res 22:228–234
Abrahamsen B, Vestergaard P, Rud B, Barenholdt O, Jensen JE, Nielsen SP, Mosekilde L, Brixen K (2006) Ten-year absolute risk of osteoporotic fractures according to BMD T score at menopause: the Danish Osteoporosis Prevention Study. J Bone Miner Res 21:796–800
De Laet C, Kanis JA, Oden A, Johanson H, Johnell O, Delmas P, Eisman JA, Kroger H, Fujiwara S, Garnero P, McCloskey EV, Mellstrom D, Melton LJ 3rd, Meunier PJ, Pols HA, Reeve J, Silman A, Tenenhouse A (2005) Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int 16:1330–1338
Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM (2001) Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA 286:2815–2822
Siris ES, Simon JA, Barton IP, McClung MR, Grauer A (2008) Effects of risedronate on fracture risk in postmenopausal women with osteopenia. Osteoporos Int 19:681–686
Seeman E, Devogelaer JP, Lorenc R, Spector T, Brixen K, Balogh A, Stucki G, Reginster JY (2008) Strontium ranelate reduces the risk of vertebral fractures in patients with osteopenia. J Bone Miner Res 23:433–438
Quandt SA, Thompson DE, Schneider DL, Nevitt MC, Black DM (2005) Effect of alendronate on vertebral fracture risk in women with bone mineral density T scores of −1.6 to −2.5 at the femoral neck: the Fracture Intervention Trial. Mayo Clin Proc 80:343–349
Vanasse A, Dagenais P, Niyonsenga T, Gregoire JP, Courteau J, Hemiari A (2005) Bone mineral density measurement and osteoporosis treatment after a fragility fracture in older adults: regional variation and determinants of use in Quebec. BMC Musculoskelet Disord 6:33
Acknowledgments
We gratefully acknowledge the contributions of the ROCQ Programme staff, particularly programme coordinators Lucie Vaillancourt and Nathalie Migneault and administrative assistant Julie Parrot. We also acknowledge the contributions of the regional coordinators and research assistants: Sylvie Bélanger, Geneviève Corneau, Isabel Lajeunesse, Pierre-Antoine Landry, Lise Lemire, Anne-Marie Louis XVI, Julie Simard and Lyse Roy. Finally, we thank the regional directors, Pierre Dagenais, Kim Latendresse, Pierre Major, Frédéric Morin, Suzanne Morin and Josée Villeneuve, for their support during the implementation of the programme and their critical scientific advice. We also thank past members of the ROCQ executive, Louise Lafortune, Christine Chin, Luc Sauriol and Andy McClenaghan, for their insightful guidance. Lastly, we appreciate all CaMos investigators for allowing us to utilise pertinent sections of the CaMos questionnaires for ROCQ. The ROCQ Programme was funded by Merck Frosst Canada, Procter and Gamble Pharmaceuticals, Sanofi-Aventis Group, Eli Lilly Canada and Novartis Pharmaceuticals Canada. None of the funding sources had a role in the collection, analysis or interpretation of the data or in the decision to publish this article.
Conflicts of interest
Drs. Bessette, Brown, Davison and Ste-Marie have consulted for, held research grants and/or received honoraria from companies that market anti-fracture therapies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bessette, L., Jean, S., Davison, K.S. et al. Factors influencing the treatment of osteoporosis following fragility fracture. Osteoporos Int 20, 1911–1919 (2009). https://doi.org/10.1007/s00198-009-0898-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-009-0898-x