Abstract
Purpose
Early noninvasive ventilation (NIV) after extubation decreases the risk of respiratory failure and lowers 90-day mortality in patients with hypercapnia. Patients with chronic respiratory disease are at risk of extubation failure. Therefore, it could be useful to determine the role of NIV with a discontinuous approach, not limited to patients with hypercapnia. We assessed the efficacy of early NIV in decreasing respiratory failure after extubation in patients with chronic respiratory disorders.
Methods
A prospective randomized controlled multicenter study was conducted. We enrolled 144 mechanically ventilated patients with chronic respiratory disorders who tolerated a spontaneous breathing trial. Patients were randomly allocated after extubation to receive either NIV (NIV group, n = 72), performed with a discontinuous approach, for the first 48 h, or conventional oxygen treatment (usual care group, n = 72). The primary endpoint was decreased respiratory failure within 48 h after extubation. Analysis was by intention to treat. This trial was registered with ClinicalTrials.gov (NCT01047852).
Results
Respiratory failure after extubation was less frequent in the NIV group: 6 (8.5%) versus 20 (27.8%); p = 0.0016. Six patients (8.5%) in the NIV group versus 13 (18.1%) in the usual care group were reintubated; p = 0.09. Intensive care unit (ICU) mortality and 90-day mortality did not differ significantly between the two groups (p = 0.28 and p = 0.33, respectively). Median postrandomization ICU length of stay was lower in the usual care group: 3 days (IQR 2–6) versus 4 days (IQR 2–7; p = 0.008). Patients with hypercapnia during a spontaneous breathing trial were at risk of developing postextubation respiratory failure [adjusted odds ratio (95% CI) = 4.56 (1.59–14.00); p = 0.006] and being intubated [adjusted odds ratio (95% CI) = 3.60 (1.07–13.31); p = 0.04].
Conclusions
Early NIV performed following a sequential protocol for the first 48 h after extubation decreased the risk of respiratory failure in patients with chronic respiratory disorders. Reintubation and mortality did not differ between NIV and conventional oxygen therapy.
Similar content being viewed by others
Introduction
The weaning/extubation period represents an essential element in the care of critically ill intubated patients receiving mechanical ventilation and an important issue for clinicians [1]. A consensus conference on weaning defined success as the absence of reintubation or noninvasive (NIV) initiation for acute respiratory failure (ARF), or death within 48 h after extubation [2, 3]. There is some evidence that weaning failure affects survival independently of the underlying illness severity [4–6]. There are many factors reported to be associated with extubation failure [1]. NIV has been recently developed for the management of postextubation ARF, the main goal being to prevent or avoid reintubation. Indeed, Ferrer et al. demonstrated that early NIV averted ARF after extubation among patients at increased risk (using the criteria identified by Epstein et al. [7]: age greater than 65 years, cardiac failure as the cause of intubation, increased severity assessed by an Acute Physiology and Chronic Health Evaluation (APACHE)—II score exceeding 12 on the day of extubation) [8]. They showed, in this same study, that early use of NIV after extubation decreased intensive care unit (ICU) mortality and improved 90-day survival in hypercapnic patients with chronic respiratory disorders [8]. Hypercapnia during the spontaneous breathing trial is independently associated with extubation failure [9] and predicts prolonged weaning and mortality [10]. Ferrer et al. furthered this issue and demonstrated that early NIV delivered for as much time as possible for a scheduled maximum period of 24 h after extubation diminished risk of respiratory failure and lowered 90-day mortality in patients with hypercapnia during a spontaneous breathing trial [11]. Nevertheless, the results of these very relevant studies raise several concerns. First, Thille et al. identified a subset of patient at high risk for extubation. There were medical patients with underlying chronic respiratory disease [5]. So it could be useful to determine the role of preventive use of NIV in patients with chronic respiratory disease and not only with hypercapnia during a spontaneous breathing trial. Second, early NIV decreased 90-day mortality without significantly decreasing reintubation rate and ICU mortality. These results deserve to be confirmed. Third, it could be interesting to evaluate or to confirm [12] the use of early NIV and at the same time a more balanced (sequential) [13] and more prolonged use (48 h).
So, we postulated that the early use of NIV performed following a sequential protocol, for the first 48 h after extubation, in patients with chronic respiratory disorders or with hypercapnia during a spontaneous breathing trial before extubation, would reduce respiratory failure after extubation. We assessed the efficacy of this strategy compared with standard medical treatment, in a randomized controlled multiple-center study, in patients who underwent a planned extubation.
Some of the results of this study have been previously reported in the form of an abstract [14].
Methods
Additional details on patients and methods are provided in the supplemental data.
An independent review board (Comité de Protection des Personnes Sud-Ouest et Outre-Mer) approved the study. This study was registered on clinicaltrials.gov with the number: NCT01047852. Written informed consent was obtained from each patient or next of kin before inclusion.
Patients
Patients were consecutively recruited between January 2010 and June 2011 from six French ICUs. Inclusion criteria were: (1) patients intubated for at least 48 h and who tolerated a spontaneous breathing trial for 2 h with a pressure support at 7 cm H2O and a positive end expiratory pressure at 0 cm H2O after recovery of their disease [15, 16]; and (2) patients with known or suspected chronic respiratory disorders, based on their clinical history (chronic respiratory disorders include chronic obstructive pulmonary disease, chronic bronchitis associated with dyspnea or former history of smoking in the absence of pulmonary function testing, bronchiectasis, sequelae of pulmonary tuberculosis, chest wall deformity or obesity associated with a restrictive ventilatory disorder), chest X-ray, arterial blood gases in steady state, and/or admission bicarbonate level, as well as previous pulmonary function tests if available [17], or those who tolerated a spontaneous breathing trial with hypercapnia defined by a \({\text{Pa}}_{{{\text{Co}}_{2} }}\) > 45 mmHg. Exclusion criteria were: facial or cranial trauma or surgery, tracheostomy, recent gastric oral or esophageal surgery, upper gastrointestinal bleeding, excessive amount of respiratory secretions, home NIV, uncooperative patient and previous decision to limit therapeutic effort in the ICU.
Study protocol
A spontaneous breathing trial (SBT) was done once the patient had reached a phase of clinical stability and the positivity criteria of the SBT were checked (see electronic supplementary material). The patients able to tolerate the SBT were extubated and those fulfilling the inclusion and non-inclusion criteria were immediately randomly allocated to one of two groups (NIV group or usual care group) via independent Web-based centralized block randomization (available 24 h/day, 7 days/week), with stratification on the center.
Except for the specific interventions of this trial, patients were managed according to the clinical protocols of their institutions.
Procedure according to the randomization arm
NIV group
After the technique was explained to the patient, NIV was delivered immediately after extubation and was applied via a face mask (ResMed, San Diego, USA) with a ventilator specifically designed for NIV or an ICU ventilator with its NIV algorithm engaged. NIV was performed in a semirecumbent position intermittently with oxygen spontaneous breathing periods, following the so-called sequential protocol, modified from the protocol proposed by Hilbert et al. [13]. NIV was not maintained continuously but used for 1 h every 3 h. The nurse and physiotherapist were asked to maintain the scheduled periods of ventilation taking into account the patient’s tolerance and trying to encourage in all cases a minimal duration of 30 min of ventilation. Concerning the night, patients in the NIV group were not woken up to perform a preventive NIV session. Conversely, ventilated patients were not woken to stop the session. In all patients, a minimal duration of 6 h by day in total of NIV will be performed. The ventilatory mode was pressure support ventilation with the addition of PEEP or bilevel positive airway pressure. Inspiratory pressure (pressure support above PEEP) was initially set to obtain an expiratory tidal volume between 6 and 8 ml/kg of predicted body weight. The PEEP was initially set at 4 cm H2O, which was titrated by 1 cm H2O increments in case of non-triggered breaths until non-triggered breaths disappeared or a maximum PEEP level of 10 cm H2O [18]. Inspiratory trigger was set at high sensitivity, pressurization slope at 200 ms. The cycling-off criteria was set at 40% of the peak inspiratory flow [19, 20] and/or the ventilator could switch from inspiration to expiration when the absolute inspiratory time was >1 s [21]. The fractional concentration of oxygen was such to achieve a \({\text{Sa}}_{{{\text{o}}_{2} }}\) > 90%. Sequential NIV was delivered for a scheduled period of 48 h, after which NIV was withdrawn and conventional oxygen treatment was administered for as long as needed.
Usual care group
Patients allocated to the usual care group received standard oxygen therapy after extubation to maintain \({\text{Sa}}_{{{\text{o}}_{2} }}\) greater than or equal to 90% [11]. Patients also received standard medical treatment, decided by the attending physicians.
Both groups received the same care by nurses and respiratory therapists during the ICU stay.
Evaluation criteria
The monitoring included an electrocardiogram, arterial oxygen saturation by pulse oximetry, invasive and/or noninvasive blood pressure and respiratory rate. We reviewed all relevant data from patients’ medical records and bedside flowcharts upon entry and at the end of the study (48 h after extubation). We extended follow-up to 90 days after randomization.
Definition of respiratory failure after extubation
Respiratory failure after extubation was defined as the presence within 48 h of extubation of at least two of the following criteria: (1) tachypnea greater than 35/min or bradypnea less than 12/min; (2) clinical signs of acute respiratory failure (i.e., cyanosis, sweating, involvement of accessory respiratory muscles, paradoxical abdominal motion, consciousness impairment); (3) hypercapnia with respiratory acidosis under nasal O2 less than or equal to 3 L/min (i.e., increase of \({\text{Pa}}_{{{\text{Co}}_{2} }}\) ≥ 10% relative to pre-extubation value [SBT] and pH ≤ 7.35); and (4) hypoxemia under O2 greater than or equal to 6 L/min or Fio 2 greater than or equal to 50% with a venturi mask (i.e., \({\text{Sa}}_{{{\text{o}}_{2} }}\) < 90% or \({\text{Pa}}_{{{\text{o}}_{2} }}\) ≤ 60 mmHg [8 kPa] or \({\text{Pa}}_{{{\text{o}}_{2} }}\)/Fio 2 ≤ 120 mmHg). We assigned causes of respiratory failure after extubation with previously published definitions [4]: upper airway obstruction, congestive heart failure, aspiration or excessive respiratory secretions (witnessed aspiration or an inability to maintain airway patency because of respiratory secretions, defined as the need for repeated nasal–tracheal aspiration or the development of atelectasis during the post-extubation period because of ineffective cough or inability to expectorate), respiratory failure, encephalopathy (decreased consciousness leading to hypoventilation).
Criteria for reintubation
For the two groups, in cases of post-extubation acute respiratory failure, the reintubation decision was based on predefined criteria (17) (see electronic supplementary material).
NIV as rescue therapy
The use of NIV was permitted as rescue therapy, before eventual reintubation, in both groups in cases of post-extubation acute respiratory failure occurrence without the need for immediate reintubation. NIV as rescue therapy was performed following a sequential protocol [18], intensified to a minimum of 1 h every 2 h and was maintained after the 48th h of post-extubation if necessary. The patients who received NIV as rescue therapy and had reintubation criteria described above, despite use of this method in optimal conditions, were reintubated.
End-points
The primary end-point was the rate of respiratory failure during the first 48 h after extubation. Secondary end-points included the need for reintubation, ICU length of stay, ICU death and mortality on day 90 after randomization.
Statistical analysis
We estimated a 30% rate of respiratory failure after extubation in patients assigned to usual care group and a rate of 10% in those assigned to the NIV group. We based the expected rate in the control group on the incidence of respiratory failure after extubation observed in patients with chronic respiratory disorders from our intensive care unit. This rate was closed to 30%. Moreover, Ferrer et al. observed a 40–48% rate of respiratory failure after extubation in more severe patients with hypercapnia [11]. We planned to include patients with chronic respiratory disease and not only with hypercapnia during a spontaneous breathing trial, i.e. less severe patients, with probably a lower risk of distress. In our experience, the incidence or respiratory failure after extubation despite use of NIV in the same population was close to 10%. In the same way, Ferrer et al. obtained a 15% rate of respiratory failure after extubation in the group treated with NIV. With the same reasoning, as for the usual care group, we expected a rate of 10% of respiratory failure in patients assigned to the NIV group. According to a two-sided 5% type 1 error and 80% power, the calculation, based on comparison of proportions, indicated a minimal sample size of 72 patients in each group, leading to a total of 144 allocated patients. The data were analyzed with SAS® base 9.1.3 (SAS Institute, Cary, NC, USA). We used the Chi-square or Fisher exact test to compare categorical variables between study groups and the unpaired Student’s t test or Mann–Whitney test to compare continuous variables. Kaplan–Meier estimate of survival curve was used to determine the cumulative 90-day survival probability. Survival curves between the two groups were compared by log-rank test. We did univariate analysis of predictors of respiratory failure after extubation with the Chi-square test and Student’s t test and multivariate analysis by logistic regression. The analyses were in intention to treat. A p value less than 0.05 was considered statistically significant.
Results
Additional details on results are provided in the supplemental data (electronic supplementary material).
Enrollment and baseline characteristics
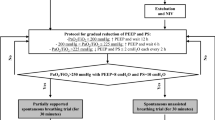
Between January 2010 and June 2011, 148 consecutive patients were registered for the study. Among them, 144 were enrolled and randomized to the usual care group (n = 72) or the NIV group (n = 72). One patient assigned to the NIV group was excluded from the data analysis because he was under guardianship. As a result, 71 and 72 patients in these two groups were kept in the primary endpoint analysis (Fig. 1).
The two groups were similar at baseline regarding general clinical characteristics and physiological variables (Table 1).
Early and sequential noninvasive ventilation
In patients assigned to NIV group, NIV was delivered for a mean period close to 8 h on day 1 and 6 h on day 2 (see electronic supplementary material, table S1). The ventilator was set with the following pressure level: 10 (8–10) cm H2O for the inspiratory pressure above PEEP and 5 (4–6) cm H2O for the positive end expiratory pressure. Mean air leak volume was 20 ± 3% of expired tidal volume. Tolerance of NIV was excellent with no specific complications associated with NIV. Only one patient developed a moderate skin breakdown [22] not requiring cessation of NIV (table S1, electronic supplementary material).
Main end points
Respiratory failure after extubation
Respiratory failure after extubation was less frequent in the NIV group compared with the usual care group (27.8 vs. 8.5%, p = 0.0016) (Table 2). This result is very close to our hypothesis for the sample size estimation. Time elapsed from extubation to respiratory failure after extubation is shown in Table 2. Most respiratory failure occurred within 1 day after extubation. Concerning the “late” respiratory failure (>48 h), four patients in the usual care group, versus six patients in the NIV group, developed a respiratory failure after extubation. However, taking into account all respiratory failure after extubation, these were less frequent in the NIV group compared with the usual care, respectively 12 (17%) versus 24 (33%), p = 0.01. After adjustment for major prognostic factors of respiratory failure (age, Apache two score, history of cardiac failure, hypercapnia), sequential and early NIV was strongly associated with a reduction in the incidence of respiratory failure within 48 h after extubation [odds ratio: 0.18; 95% confidence interval (0.05–0.55); p = 0.004] (Table 3).
Reintubation
In patients who developed respiratory failure after extubation, three patients in each group required immediate reintubation. For others, NIV as rescue therapy resulted in avoidance of reintubation in 7 of 17 patients assigned in the usual care group, but was not effective in the NIV group (Fig. 1). The reintubation rate was not significantly lower in the NIV group (Table 2). In univariate and multivariate analysis, patients with hypercapnia and with an Apache II > 12 were at risk of reintubation (Table 3).
Mortality
Mortality in the ICU was three times more frequent in the usual care group, as compared with NIV Group; however, this difference was not significant [6 (8.3%) vs. 2 (2.8%), p = 0.28]. Mortality at 90 days was not significantly different between the two groups (Fig. 2). Table 2 shows causes of death within 90 days of randomization. In univariate and multivariate analysis, only patients with an Apache II > 12 were at risk of 90-days mortality (Table 3).
Subgroup analysis
In patients without hypercapnia, in multivariate analysis, the randomization group was not a predictor of respiratory failure after extubation with adjusted odds ratio (95% confidence interval) = 0.15 (0.01–1.28), p = 0.08 (Table 3). We observed the same results in patient with hypercapnia (Table 3).
Patient with hypercapnia during a spontaneous breathing trial were at risk of developing post-extubation respiratory failure [adjusted odds ratio (95% CI) = 4.56 (1.59–14.00); p = 0.006] and being intubated [adjusted odds ratio (95% CI) = 3.60 (1.07–13.31); p = 0.04].
Discussion
In this randomized controlled trial, a sequential and early use of NIV was associated with a significant reduction of post-extubation respiratory failure in patients with chronic respiratory disorders. However, this strategy did not result in lowered mortality or reintubation in our population.
Patients at risk of post-extubation respiratory failure
One important research objective regarding the indication for early use of NIV immediately after extubation in the absence of evidence of respiratory failure is to identify the high-risk population of patients most likely to benefit from this intervention [1]. This “prophylactic” NIV seems beneficial only in patients at high risk for reintubation. Indeed, in a study including more than 400 ICU patients extubated after a successful 2-h spontaneous breathing trial, extubation failure rate was similar in patients treated with early NIV or oxygen therapy [23]. The result of this study suggests that their patients in general were at lower risk for extubation failure than those in the studies of Nava et al. [12] and Ferrer et al. [8, 11]. In addition, the composition of their patient population, with fewer patients with COPD, may also explain the lack of efficacy. The existing data suggest that early use of NIV after extubation might be indicated in patients older than 65 years, high severity score, heart failure [7] or in hypercapnic patients [8, 11]. However, patients with underlying chronic respiratory disease are identified as being at high risk for extubation failure [11]. For patients at high risk for extubation failure (patients with hypercapnia, COPD, chronic heart failure, or other serious comorbidities) who have been receiving mechanical ventilation for more than 24 h, and who have passed an SBT, an official clinical practice guideline from the American College of Chest Physicians and from the American Thoracic Society recommend extubation to preventive NIV [24]. Our study confirms these data and seems to indicate that nearly 30% of patients with chronic respiratory disorders, not exclusively hypercapnic, will develop respiratory failure after extubation. Another strength of the present trial is to confirm the benefits of prophylactic NIV in these patients. However, a subgroup analysis shown that, in patients without hypercapnia, the randomization group was not a predictor of respiratory failure after extubation in multivariate analysis. We observed the same results in patients with hypercapnia. This can be explained by a lack of power.
Mortality
In our study, neither ICU mortality rate nor the 90-day mortality rate were reduced with NIV. The reintubation rate of each group was similar compared with the difference in rate of respiratory failure after extubation between groups suggesting a protective effect of NIV as rescue therapy. In the study performed by Ferrer et al. in hypercapnic patients, the 90-day mortality rate was reduced with NIV. The authors provide several relevant explanations: only few patients from the NIV group needed reintubation; differences in mortality between groups arose later after discharge from the ICU, suggesting a long-term benefit of NIV [25]. We have not observed the same results. Our patients are likely to be less severe, not exclusively hypercapnic. Indeed, hypercapnia is associated with poor survival in mechanically ventilated patients particularly when no ventilatory support is provided after extubation [26, 27]. Another explanation, probably the most logical, is the fortuitously low number of deaths.
Reintubation
In our study, reintubation did not differ significantly between groups. Indeed, patients who did not require immediate reintubation escaped from reintubation after NIV was used as rescue therapy. Thille et al. performed a recent before–after study to assess the impact of a prophylactic NIV protocol on reintubation in a population of at-risk patients [28]. The reintubation rate was significantly decreased from 28% in the control cohort to 15% in the NIV cohort. However, as expressed by the authors, many changes may have impacted the reintubation rate such an improvement in the process of weaning or the use of NIV as rescue therapy.
NIV as recue therapy
There is some evidence that reintubation and prolongation of mechanical ventilation adversely affect survival independently of the underlying illness severity [6, 11]. In a large case-series study of planned extubation, Fructos-Vivar et al. showed that reintubation was independently associated with ICU mortality [6]. Results are disappointing in patients in whom NIV was used as rescue therapy, i.e. after they developed respiratory failure [29–31]. In our study, 20 patients, who developed respiratory failure after extubation but who did not need immediate reintubation, received NIV as rescue therapy. This strategy makes sense, because it has been consistently shown that patients with chronic pulmonary disease benefit from NIV [18, 32]. However, this remains a high-risk situation. Indeed, NIV used to avoid reintubation was successful in a few patients (7/17) in the usual care group and ineffective in the preventive NIV group (0/3). We can reasonably assume that some patients would have been reintubated for respiratory failure after extubation if NIV was not applied as rescue therapy. These positive results with NIV as rescue therapy confirm those previously reported, particularly in patients with chronic respiratory disease [8, 11, 17], although reintubation after rescue therapy with NIV occurred later than direct reintubation in our study. ICU mortality rate of patients who were reintubated directly (3/6 patients) was similar to that of patients who were reintubated after failure of rescue therapy with NIV (5/13 patients).
Sequential use of NIV
Most of the studies about preventive use of NIV after extubation performed the technique in a continuous way during a duration from 12 to 24 h [8, 11, 23, 33, 34]. In those studies, the facial skin was assessed regularly every 4 h to prevent damage from the face mask. Although in these studies NIV’ tolerance was satisfactory, in the study performed by Ferrer et al., 10% of patients assigned to continuous NIV for 24 h after extubation tolerated the procedure for only 6 h or less [11]. Some authors have emphasized that NIV is not easily accepted by patients with acute respiratory failure [35, 36]. This explains at least partly why in many studies the objectives of either continuous ventilation or prolonged periods of ventilation have not been achieved. We chose a different strategy, because NIV was applied following the so-called sequential protocol proposed by Hilbert et al. [13]. All the goals of NIV can also be achieved by a sequential use, and in our study tolerance of NIV was excellent with no specific complication.
Duration of NIV and late post-extubation failure
In ARF in patients with chronic respiratory disease, the mean number of hours of treatment per day is close to 8 h [37–39]. In most of the studies about the preventive use of NIV after extubation, the number of hours of treatment was paradoxically much more important, even though it is a preventive process in patients who are not in ARF. Our protocol of ventilation is appreciated by our nursing staff and it has not been necessary to modify the organization of our unit. Another point to discuss is the interest to perform NIV for 48 h in post-extubation. This attitude make sense, because roughly 20% of those patients who developed a respiratory failure did so on the second day after extubation. However, only four patients in each group developed respiratory failure the second day after extubation. Taking into account the small sizes of those subgroups, it is difficult to conclude to the interest to perform NIV for 48 h in post-extubation. Nevertheless, the tolerance to NIV was high and the rate of complications low, in keeping with our previous experiences. Giving the fact that NIV was maintained for 2 days, its suspension may have caused a relapse of acute respiratory failure. Indeed six patients in the NIV group versus four in the usual care group developed a late respiratory failure. It seemed difficult to compare those data. In addition, it could be questionable to establish a link between late failure and extubation.
High flow nasal cannula
Another point to discuss is the role of high-flow nasal cannula at the light of the recent randomized trials. Among extubated patients at low risk for reintubation, the use of high-flow nasal cannula oxygen compared with conventional oxygen therapy reduced the risk of reintubation [40]. In patients at risk of post-extubation respiratory failure, Hernandez et al. recently performe a multicenter randomized clinical trial to test if high-flow oxygen therapy was noninferior to NIV for preventing post-extubation respiratory failure [41]. High-flow nasal cannula was not inferior to NIV for risk of reintubation. As noted by the authors, one possible limitation is the criteria used to select patients who were at high risk of reintubation. In fact, it could explain the very high and unusual percentage (close to 40%) of post-extubation respiratory failure in the NIV group.
Limitations of the study
Several limitations of our study should be acknowledged. It was not possible to conduct a double-blind investigation. Despite clearly predefined evaluation criteria, interventional procedure and clinical decisions during the trial, it was not possible to entirely control this bias. The use of rescue NIV in cases of post-extubation ARF might have contributed to reduce the difference in patient outcomes between the two groups. Nevertheless, it seemed difficult not to offer to the patients the opportunity of rescue NIV before having to reintubate. Patients in the usual care group presented more underlying diseases than in the NIV group. Nevertheless, there was no significant statistical difference. This lack of difference could be due to the small size of both populations. The present study was conducted in centers with experience with NIV. This factor could have affected the success of this technique. In our opinion, a skilled staff is crucial to apply the technique with efficiency and security in the post-extubation period.
Conclusion
Early NIV performed following a sequential protocol, for the first 48 h after extubation, decreases risk of respiratory failure in patients with chronic respiratory disorders. However, the rate of reintubation and mortality did not change between groups. A broader future study should be performed to try to demonstrate stronger benefits of NIV after extubation in patients with chronic respiratory disorders. This trial confirms the effectiveness of NIV in this clinical setting. Our result should support the implementation of NIV in clinical practice to manage the post-extubation period in these patients.
References
Thille AW, Richard JC, Brochard L (2013) The decision to extubate in the intensive care unit. Am J Respir Crit Care Med 187:1294–1302
Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, Pearl R, Silverman H, Stanchina M, Vieillard-Baron A, Welte T (2007) Weaning from mechanical ventilation. Eur Respir J 29:1033–1056
Perren A, Previsdomini M, Llamas M, Cerutti B, Györik S, Merlani G, Jolliet P (2010) Patients’ prediction of extubation success. Intensive Care Med 36:2045–2052
Epstein SK, Ciubotaru RL (1998) Independent effects of etiology of failure and time to reintubation on outcome for patients failing extubation. Am J Respir Crit Care Med 158:489–493
Thille AW, Harrois A, Schortgen F, Brun-Buisson C, Brochard L (2011) Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med 39:2612–2618
Frutos-Vivar F, Esteban A, Apezteguia C, Gonzalez M, Arabi Y, Restrepo MI, Gordo F, Santos C, Alhashemi JA, Perez F, Penuelas O, Anzueto A (2011) Outcome of reintubated patients after scheduled extubation. J Crit Care 26:502–509
Epstein SK, Ciubotaru RL, Wong JB (1997) Effects of failed extubation on the outcome of mechanical ventilation. Chest 112:186–192
Ferrer M, Valencia M, Nicolas JM, Bernadich O, Badia JR, Torres A (2006) Early noninvasive ventilation averts extubation failure in patients at risk: a randomized controlled trial. Am J Respir Crit Care Med 173:164–170
Mokhlesi AM, Tulaimat A, Gluckman TJ, Wang Y, Evans AT, Corbridge TC (2007) Predicting extubation failure after successful completion of a spontaneous breathing trial. Respir Care 52:1710–1717
Sellares J, Ferrer M, Cano E, Loureiro H, Valencia M, Torres A (2011) Predictors of pronlonged weaning and survival during ventilator weaning in a respiratory ICU. Intensive Care Med 37:775–784
Ferrer M, Sellares J, Valencia M, Carrillo A, Gonzalez G, Badia JR, Nicolas JM, Torres A (2009) Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: randomised controlled trial. Lancet 374:1082–1088
Nava S, Gregoretti C, Fanfulla F, Squadrone E, Grassi M, Carlucci A, Beltrame F, Navalesi P (2005) Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med 33:2465–2470
Hilbert G, Gruson D, Gbikpi-Benissan G, Cardinaud JP (1997) Sequential use of noninvasive pressure support ventilation for acute exacerbations of COPD. Intensive Care Med 23:955–961
Vargas F, Clavel M, Sanchez P, Garnier S, Boyer A, Bui HN, Kerchache A, Guisset A, Benard A, Asselineau J, Clouzeau B, Gruson D, Vignon P, Hilbert G (2012) Sequential and early used of noninvasive ventilation after extubation in patients with chronic respiratory disorders. Am J Respir Crit Care Med 185:A6487
Esteban A, Alia I, Gordo F, Fernandez R, Solsona JF, Vallverdu I, Macias S, Allegue J, Blanco J, Carriedo D, Leon M, Taboada F, Gonzalez de Velasco J, Palazon E, Carrizosa F, Tomas R, Suarez J, Goldwasser RS (1997) Extubation outcome after spontaneous breathing trials with T-Tube or pressure support ventilation. Am J Respir Crit Care Med 156:459–465
Perren A, Domenighetti G, Mauri S, Genini F, Vizzardi N (2002) Protocol-directed weaning from mechanical ventilation: clinical outcome in patients randomized for a 30-min or 120-min trial with pressure support ventilation. Intensive Care Med 28:1058–1063
Girault C, Bubenheim M, Abroug F, Diehl JL, Elatrous S, Beuret P, Richecoeur J, L’Her E, Hilbert G, Capellier G, Rabbat A, Besbes M, Guerin C, Guiot P, Benichou J, Bonmarchand G, VENISE Trial Group (2011) Noninvasive ventilation and weaning in patients with chronic hypercapnic respiratory failure. Am J Respir Crit Care Med 184:672–679
Hilbert G, Gruson D, Portel L, Gbikpi-Benissan G, Cardinaud JP (1998) Noninvasive pressure support ventilation in COPD patients with postextubation hypercapnic respiratory insufficiency. Eur Respir J 11:1349–1359
Tassaux D, Gainnier M, Battisti A, Jolliet P (2005) Impact of expiratory trigger setting on delayed cycling and inspiratory muscle workload. Am J Respir Crit Care Med 172:1283–1289
Chiumello D, Polli F, Tallarini F, Chierichetti M, Motta G, Azzari S, Colombo R, Rech R, Pelosi P, Raimondi F, Gattinoni L (2007) Effect of different cycling-off criteria and positive end expiratory pressure during pressure support ventilation in patients with chronic obstructive pulmonary disease. Crit Care Med 35:2547–2552
Calderini E, Confalonieri M, Puccio PG, Francavilla N, Stella L, Gregoretti C (1999) Patient-ventilator asynchrony during noninvasive ventilation: the role of expiratory trigger. Intensive Care Med 25:662–667
Gregoretti C, Confalonieri M, Navalesi P, Squadrone V, Frigerio P, Beltrame F, Carbone G, Conti G, Gamma F, Nava S, Calderini E, Skrobik Y, Antonelli M (2012) Evaluation of patient skin breakdown and comfort with a new face mask for non-invasive ventilation: a multi-center study. Intensive Care Med 28:278–284
Su CL, Chiang LL, Yang SH, Lin HI, Cheng KC, Huang YC, Wu CP (2012) Preventive use of noninvasive ventilation after extubation: a prospective, multicenter randomized controlled trial. Respir Care 57:204–210
Ouellette DR, Patel S, Girard TD, Morris PE, Schmidt GA, Truwit JD, Alhazzani W, Burns SM, Epstein SK, Esteban A, Fan E, Ferrer M, Fraser GL, Gong MN, Hough CL, Mehta S, Nanchal R, Pawlik AJ, Schweickert WD, Sessler CN, Strom T, Kress JP (2017) Liberation from mechanical ventilation in critically ill alduts: On Official American College of Chest Physicians/American Thoracic Society clinical practice guideline: inspiratory pressure augmentation during spontaneous breathing trials, protocols minimizing sedation, and noninvasive ventilation immediately after extubation. Chest 151:166–180
Confalonieri M, Parigi P, Scartabellati A, Aiolfi S, Scorsetti S, Nava S, Gandola L (1996) Noninvasive mechanical ventilation improves the immediate and long-term outcome of COPD patients with acute respiratory failure. Eur Respir J 9:422–430
Ferrer M, Esquinas A, Arancibia F, Bauer TT, Gonzalez G, Carrillo A, Rodriguez-Roisin R, Torres A (2003) Noninvasive ventilation during persistent weaning failure: a randomized controlled trial. Am J Respir Crit Care Med 168:70–76
Carlucci A, Delmastro M, Rubini F, Fracchia C, Nava S (2003) Changes in the practice of non-invasive ventilation in treating COPD patients over 8 years. Intensive Care Med 29:419–425
Thille AW, Boissier F, Ben-Ghezala H, Razazi K, Mekontso-Dessap A, Brun-Buisson C, Brochard L (2016) Easily identified at-risk patients for extubation failure may benefit from noninvasive ventilation: a prospective before-after study. Crit Care 20:48. doi:10.1186/s13054-016-1228-2
Keenan SP, Sinuff T, Burns KE, Muscedere J, Kutsogiannis J, Mehta S, Cook DJ, Ayas N, Adhikari NK, Hand L, Scales DC, Pagnotta R, Lazosky L, Rocker G, Dial S, Laupland K, Sanders K, Dodek P, Canadian Critical Care Trials Group/Canadian Critical Care Society Noninvasive Ventilation Guidelines Group (2011) Clinical practice guidelines for the use on noninvasive positive pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ 183:E195–E214
Keenan SP, Powers C, McCormack DG, Block G (2002) Noninvasive positive-pressure ventilation for postextubation respiratory distress: a randomized controlled trial. JAMA 287:3238–3244
Esteban A, Frutos-Vivar F, Ferguson ND, Arabi Y, Apeztequia C, Gonzalez M, Epstein SK, Hill NS, Nava S, Soares MA, D’empaire G, Alia I, Anzueto A (2004) Noninvasive positive pressure ventilation for respiratory failure after extubation. N Engl J Med 350:2452–2460
Peter JV, Moran JL, Phillips-Hughes J, Warn D (2002) Noninvasive ventilation in acute respiratory failure-A meta-analysis update. Crit Care Med 30:555–562
Ornico SR, Lobo SM, Sanches HS, Deberaldini M, Tofoli LT, Vidal AM, Schettino GP, Amato MB, Carvalho CR, Barbas CS (2013) Noninvasive ventilation immediately after extubation improves weaning outcome after acute respiratory failure: a randomized controlled trial. Crit Care 17:R39
Bajaj A, Rathor P, Sehgal V, Shetty A (2015) Efficacy of noninvasive ventilation after planned extubation: A systematic review and meta-analysis of randomized controlled trials. Heart Lung 44:150–157
Foglio C, Vitacca M, Quadri A, Scalvini S, Marangoni S, Ambrosino N (1992) Acute exacerbation in severe COLD patients. Treatment using positive pressure ventilation by nasal mask. Chest 101:1533–1538
Chevrolet JC, Jolliet P, Abajo B, Toussi A, Louis M (1991) Nasal positive pressure ventilation in patients with acute respiratory failure. Difficult and time-consuming procedure for nurses. Chest 100:775–782
Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, Simonneau G, Benito S, Gasparetto A, Lemaire F et al (1995) Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 333:817–822
Lightowler JV, Wedzicha JA, Elliot NW, Ram FS (2003) Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbation of chronic obstructive pulmonary disease: cochrane systematic review and meta-analysis. BMJ 326(7382):185
Quon BS, Gan WQ, Sin DD (2008) Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest 133:756–766
Hernandez G, Vaquero C, Gonzales P, Subira C, Fructos-Vivar F, Rialp G, Laborda C, Colinas L, Cuena R, Fernandez R (2016) Effects of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients A randomized clinical trial. JAMA 315:1354–1361
Hernandez G, Vaquero C, Colinas L, Cuena R, Gonzalez P, Canabal A, Sanchez S, Rodriguez L, Villasclaras A, Fernandez R (2016) Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients. A randomized clinical trial. JAMA 316:1565–1574
Acknowledgements
We are grateful to the junior doctors, nursing staff, and our clinical research associate Marie-Pierre Baudier. We thank Patrick McSweeny for stylistic editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This trial was supported and promoted by the French publicly funded hospital clinical research program (programme hospitalier de recherche clinique).
Conflicts of interest
The authors do not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Ethical approval
All procedures performed were in accordance with the ethical standards of our institutional research committee. An independent review board (Comité de protection des Personnes Sud-Ouest et Outre Mer III) approved the study.
Informed consent
Written informed consent was obtained from each patient or next of skin before inclusion.
Additional information
Take-home message
Intermittent noninvasive ventilation for a period of 48 h early after extubation at the rate of at least 6 h per day is more effective than standard medical therapy in preventing postextubation respiratory failure in patients with chronic respiratory disorders. Our findings suggest that NIV is well tolerated and effective in a population considered at risk of developing this complication, and the benefits are not limited to patients exhibiting hypercapnia during a spontaneous breathing trial.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vargas, F., Clavel, M., Sanchez-Verlan, P. et al. Intermittent noninvasive ventilation after extubation in patients with chronic respiratory disorders: a multicenter randomized controlled trial (VHYPER). Intensive Care Med 43, 1626–1636 (2017). https://doi.org/10.1007/s00134-017-4785-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-017-4785-1