Abstract
Purpose
Pressure support is often used for extubation readiness testing, to overcome perceived imposed work of breathing from endotracheal tubes. We sought to determine whether effort of breathing on continuous positive airway pressure (CPAP) of 5 cmH2O is higher than post-extubation effort, and if this is confounded by endotracheal tube size or post-extubation noninvasive respiratory support.
Methods
Prospective trial in intubated children. Using esophageal manometry we compared effort of breathing with pressure rate product under four conditions: pressure support 10/5 cmH2O, CPAP 5 cmH2O (CPAP), and spontaneous breathing 5 and 60 min post-extubation. Subgroup analysis excluded post-extubation upper airway obstruction (UAO) and stratified by endotracheal tube size and post-extubation noninvasive respiratory support.
Results
We included 409 children. Pressure rate product on pressure support [100 (IQR 60, 175)] was lower than CPAP [200 (120, 300)], which was lower than 5 min [300 (150, 500)] and 60 min [255 (175, 400)] post-extubation (all p < 0.01). Excluding 107 patients with post-extubation UAO (where pressure rate product after extubation is expected to be higher), pressure support still underestimated post-extubation effort by 126–147 %, and CPAP underestimated post-extubation effort by 17–25 %. For all endotracheal tube subgroups, ≤3.5 mmID (n = 152), 4–4.5 mmID (n = 102), and ≥5.0 mmID (n = 48), pressure rate product on pressure support was lower than CPAP and post-extubation (all p < 0.0001), while CPAP pressure rate product was not different from post-extubation (all p < 0.05). These findings were similar for patients extubated to noninvasive respiratory support, where pressure rate product on pressure support before extubation was significantly lower than pressure rate product post-extubation on noninvasive respiratory support (p < 0.0001, n = 81).
Conclusions
Regardless of endotracheal tube size, pressure support during extubation readiness tests significantly underestimates post-extubation effort of breathing.
Similar content being viewed by others
Introduction
Critical care practitioners commonly add pressure support during extubation readiness tests to overcome perceived imposed resistance of the endotracheal tube [1–3]. A recent survey of pediatric critical care practitioners identified that 94 % use pressure support when performing extubation readiness tests [4]. There is a reluctance to wean patients to continuous positive airway pressure (CPAP) alone (without pressure support), or to T-piece ventilation, on the basis of the hypothesis that patients work harder to breathe on CPAP than they will extubated because of small endotracheal tube diameters (smaller than the trachea) and imposed resistance from ventilator circuits [2]. This perception is amplified because the internal diameter of the endotracheal tubes is often reduced by secretions or biofilm, thereby further increasing airway resistance [5–7].
However, resistance through a tube depends on flow [8]. Previous in vitro work has demonstrated that resistance of the smallest endotracheal tubes is higher when matched for flow (i.e., 15 L/min through a 3.5-mmID tube has higher resistance than 15 L/min through a 6.0-mmID tube), but children with 3.5-mmID endotracheal tubes generally breathe at lower flow rates than those who have 6.0-mmID endotracheal tubes [9]. Several studies demonstrate that pressure support or automatic endotracheal tube compensation overcomes imposed resistance from the endotracheal tube or ventilator circuits [3, 5, 11–14]. However, these studies do not factor in the resistance of the natural airway and have not routinely measured the difference between effort of breathing before and after extubation. There are limited controlled data in adults demonstrating that extubation readiness tests using automatic tube compensation or pressure support result in higher rates of successful extubation compared to CPAP or T-piece [15–17], and no pediatric data. The increasing use of noninvasive respiratory support after extubation complicates this picture, as it is unclear if using noninvasive respiratory support after extubation leads to patients to being extubated from higher pressure support because of a belief that the noninvasive support can further lower effort of breathing after extubation.
While we have previously demonstrated that pressure support underestimates post-extubation effort, our findings were limited by small sample size [18]. We hypothesized that effort of breathing on CPAP of 5 cmH2O was not higher than post-extubation and that using pressure support prior to extubation would significantly underestimate post-extubation effort. We tested this hypothesis by comparing effort of breathing for children before extubation on pressure support/CPAP and CPAP alone to effort of breathing post-extubation. We, a priori, sought to control for post-extubation upper airway obstruction (UAO) and stratify by size of endotracheal tube and use of noninvasive respiratory support after extubation. Some of these findings have been published in abstract form [19].
Methods
We screened intubated children in the pediatric or cardiothoracic intensive care units at the Children’s Hospital Los Angeles from July 2012 to April 2015. Inclusion criteria were greater than 37 weeks gestational age to 18 years, intubated for at least 12 h with extubation from 7 am to 5 pm Monday–Friday. Exclusion criteria were contraindication to esophageal catheter or respiratory inductance plethysmography bands. Informed consent was obtained, with approval from the institutional review board, and the study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. This was a planned secondary analysis to a study on post-extubation UAO containing further details about methods [20]. Pressure support recordings were added to the UAO study protocol to test this hypothesis.
Study protocol
Prior to extubation, each child received an esophageal balloon catheter and had respiratory inductance plethysmography bands calibrated under tidal breathing on CPAP of 5 cmH2O [21, 22]. A self-calibrating pneumotachometer was used prior to extubation to measure peak inspiratory flow during tidal breathing.
When the clinical team determined that the patient was ready for extubation, data were recorded under four conditions in the following order: pressure support 10/PEEP 5 cmH2O, CPAP 5 cmH2O (CPAP), and spontaneous breathing, 5 min and 60 min after extubation. Pressure support and CPAP measurements were within 20 min of extubation. During pressure support, the research respiratory therapist screened for autotriggering and missed triggers by comparing esophageal manometer tracings with spirometry and when necessary adjusted trigger sensitivity. Effort of breathing was allowed to stabilize for up to 5 min on a given condition, at which point we recorded data during 5 min of steady state breathing and calculated the median pressure rate product = peak-to-trough change in esophageal pressure (cmH2O) × respiratory rate (breaths per minute) over the entire 5 min, after removing artifacts. Pressure rate product is a surrogate for effort of breathing [23–26], and unlike work of breathing (calculated from the pressure–volume curve) pressure rate product does not need to be subdivided into patient versus ventilator effort. All of the pressure rate product is attributable to the patient, making it appropriate for comparing patient effort on and off mechanical ventilation. While pressure time product can also be used, pressure rate product is easier to calculate and in our experience more robust against artifacts, particularly in young children.
If the patient was initiated on noninvasive respiratory support within 60 min of extubation (high flow humidified nasal cannula, nasal intermittent mandatory ventilation, CPAP, or bi-level positive airway pressure), additional measurements were obtained 15–20 min after noninvasive respiratory support initiation. One of two ventilators was used for pressure support and CPAP: SERVO-I ® (MAQUET) and AVEA® (Carefusion). Automatic endotracheal tube compensation is available on the AVEA®, but was off during measurements.
UAO (either subglottic or supraglottic) was labeled as present after extubation when there was inspiratory flow limitation on the plot of flow from calibrated respiratory inductance plethysmography and esophageal pressure, as previously described [20]. Average peak inspiratory flow (L/min) was measured from spirometry during 2–3 min of steady state breathing on CPAP of 5 cmH2O. Peak inspiratory resistance (cmH2O/L/s) was calculated using the patient’s endotracheal tube size and measured peak inspiratory flow, using formulae derived from in vitro studies [9].
Analysis
We hypothesized that effort of breathing on CPAP of 5 cmH2O was not higher than post-extubation effort and attempting to “overcome endotracheal tube resistance” with pressure support would underestimate post-extubation effort of breathing. Secondary objectives were to determine whether this relationship was confounded by size of endotracheal tube or post-extubation noninvasive respiratory support. For all subgroups, we excluded patients with post-extubation UAO because post-extubation pressure rate product is expected to be significantly higher than pre-extubation pressure rate product for patients with UAO, and including these patients may bias results in favor of our hypothesis. Three ranges of endotracheal tube sizes were used for subgroup analysis (≤3.5 mmID, 4.0–4.5 mmID, and ≥5.0 mmID), ensuring sufficient patients per group for analysis. Separate subgroup analysis was performed for patients on noninvasive respiratory support within 1 h of extubation, comparing their pre-extubation pressure rate product with pressure rate product 15–20 min after noninvasive respiratory support initiation. Descriptive statistics are reported using median (interquartile range) or number (percent) as well as box and whisker plots. Pressure rate product was log transformed for repeated measures analysis of variance (ANOVA) with Scheffe’s test for post hoc multiple comparisons. Statistical analysis was performed in Statistica 10 (Dell, Tulsa, Oklahoma) and Stata 10 (StataCorp, College Station, Texas). Overall sample size was determined by the UAO study. However, a priori power analysis demonstrated that we could detect a 25 % difference in pressure rate product, which we considered clinically significant, with an alpha of 0.05, a power goal of 80 %, with a minimum sample size of 45 patients (in any subgroup).
Results
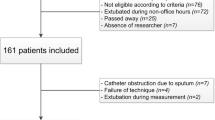
A total of 1159 patients were eligible and 409 were included, with a median age of 5 months (IQR 1, 16) and 58 % male. The most common reasons eligible patients were not enrolled were (a) parents unavailable for consent and (b) extubation when the study team was unavailable (night or weekend). Demographics and reasons for non-enrollment have been previously published [20]. Approximately half were intubated for cardiac surgery. Median length of mechanical ventilation was 4.1 days (IQR 1.4, 8.0 days). Thirty-four patients were reintubated (8.3 %), and 107 (26 %) had post-extubation UAO (subglottic or supraglottic) within 1 h of extubation. One hundred and seven (26 %) children were on noninvasive respiratory support within 1 h of extubation (mostly high flow nasal cannula) (Table 1).
Resistance as a function of flow and endotracheal tube size
For the entire cohort, 209 (51 %) had an endotracheal tube ≤3.5 mmID, 143 (35 %) had a tube of 4.0 or 4.5 mmID, and 57 (14 %) had a tube of ≥5.0 mmID (Table 1). Peak inspiratory flow increased as endotracheal tube size increased (Fig. 1). While peak inspiratory resistance was higher for smaller endotracheal tubes, median peak inspiratory resistance was lower in intubated children compared to expected values while extubated, even with a 3.0-mmID endotracheal tube on CPAP of 5 cmH2O [27–30] (Fig. 1). The flow rates used by most infants and children while intubated are in a range in which significant increases in resistance are not expected (Fig. 2).
Peak inspiratory flow (dotted) (L/min) and peak inspiratory resistance (solid) (cmH2O/L/s) as a function of endotracheal tube size. Note, 2.5-mmID endotracheal tube was excluded from graph because there were only 3 measurements. 6.0-mmID endotracheal tube includes all those with endotracheal tubes ≥6.0 mmID. While resistance is nearly 3-fold higher for 3.0-mmID compared to 6.0-mmID endotracheal tubes, these values are lower than peak resistance with a natural airway
Range of peak resistance (cmH2O/L/s) expected on the basis of actual patient peak inspiratory flow (L/min) superimposed on bench model of endotracheal tube resistance from Manczur et al. [9]. The shaded areas for each endotracheal tube represent the minimum to maximum observed patient flow on CPAP of 5 cmH2O prior to extubation with predicted resistance. Note that for patients with small endotracheal tubes, flow is lower, predicting resistances that are still lower than expected with a natural airway
Effort of breathing before and after extubation
For the entire cohort (n = 409), median pressure rate product was 100 (IQR 60, 175) on pressure support 10/5 cmH2O, 200 (IQR 120, 300) on CPAP of 5 cmH2O, 300 (IQR 150, 500) 5 min after extubation, and 255 (IQR 175, 400) 60 min after extubation (Fig. 3). Pressure support pressure rate product was lower than CPAP and post-extubation pressure rate product (multiple comparisons p < 0.0001), and CPAP pressure rate product was lower than post-extubation pressure rate product (multiple comparisons p < 0.0001). Restricting the analysis to patients without post-extubation UAO (n = 302), there were similar trends (Fig. 4). Again, pressure support pressure rate product was lower than CPAP and post-extubation pressure rate product (multiple comparisons p < 0.0001), and CPAP pressure rate product was lower than post-extubation pressure rate product (multiple comparisons p < 0.02).
Pressure rate product as a function of pre-extubation support (pressure support of 10/PEEP of 5 cmH2O), CPAP (continuous positive airway pressure = 5 cmH2O), and spontaneously breathing 5 and 60 min post-extubation, for the entire cohort of 409 children. All pressure rate product values were significantly different from one another, with pressure rate product on pressure support < CPAP < post-extubation (log transformed pressure rate product, ANOVA p < 0.0001, multiple comparisons all p < 0.0001)
Pressure rate product as a function of pre-extubation support (pressure support of 10/PEEP of 5 cmH2O), CPAP (continuous positive airway pressure = 5 cmH2O), and spontaneously breathing 5 and 60 min post extubation, excluding the 107 patients with post-extubation UAO. All pressure rate product values were significantly different from one another with pressure rate product on pressure support < CPAP < post-extubation (log transformed pressure rate product, ANOVA p < 0.0001, multiple comparisons all p < 0.02)
Excluding patients with UAO, 5 min after extubation an individual patient’s pressure rate product was a median 25 % (IQR −5, 72 %) higher than CPAP values and a median 147 % (67, 267 %) higher than pressure support values. By 60 min after extubation, an individual patient’s pressure rate product remained a median 17 % (−20, 60 %) higher than CPAP values and a median 126 % (40, 233 %) higher than pressure support values.
Subgroup analysis: endotracheal tube size and noninvasive respiratory support
When children without UAO were subgrouped by endotracheal tube size, patterns were similar for endotracheal tube size groupings of ≤3.5 mmID (n = 152, Fig. 5a), 4–4.5 mmID (n = 102, Fig. 5b), and ≥5.0 mmID (n = 48, Fig. 5c). Regardless of endotracheal tube size subgroup, pressure support pressure rate product was less than CPAP and post-extubation pressure rate product (all multiple comparisons p < 0.01), while within each endotracheal tube subgroup, CPAP pressure rate product was similar to post-extubation pressure rate product (all multiple comparisons p > 0.05).
Pressure rate product as a function of pre-extubation support (pressure support of 10/PEEP of 5 cmH2O), CPAP (continuous positive airway pressure = 5 cmH2O), and spontaneously breathing 5 and 60 min post-extubation, excluding the 107 patients with post-extubation UAO, stratified by endotracheal tube size. The patterns were the same for endotracheal tube size groupings of a ≤3.5 mmID (n = 152), b 4–4.5 mmID (n = 102), and c ≥5.0 mmID (n = 48). Regardless of endotracheal tube subgrouping, pressure rate product on pressure support was less than CPAP (log transformed pressure rate product, ANOVA p < 0.0001, multiple comparisons all p < 0.01). CPAP pressure rate product was similar to post-extubation pressure rate product (p > 0.05)
When we examined the cohort of 107 children on noninvasive respiratory support within 1 h of extubation, 26 had post-extubation UAO. In the remaining 81 patients (2 bi-level positive airway pressure, 74 high flow humidified nasal cannula, 5 nasal intermittent mandatory ventilation), the median pressure rate product on pressure support prior to extubation [135 (IQR 90, 220)] was significantly lower than the pressure rate product on either CPAP prior to extubation [270 (200, 400)] or post-extubation on noninvasive respiratory support [320 (220, 420)] (multiple comparisons p < 0.0001). However there was no difference in pressure rate product on CPAP prior to extubation and pressure rate product post-extubation on noninvasive respiratory support (multiple comparisons p = 0.8).
Discussion
We have demonstrated that pressure support during extubation readiness tests significantly underestimates post-extubation effort of breathing, regardless of endotracheal tube size or use of noninvasive respiratory support (mostly high flow nasal cannula) after extubation. Effort of breathing on CPAP of 5 cmH2O may still underestimate post-extubation effort, although on average it is 15–25 % lower than post-extubation and appears similar to effort of breathing on noninvasive respiratory support after extubation. In other words, if patient effort of breathing is high on CPAP 5 cmH2O prior to extubation, they are unlikely to do well after extubation, even with noninvasive respiratory support. While inspiratory resistance increases as endotracheal tube size decreases, prior to extubation children are breathing with flow rates where predicted inspiratory resistance is actually below extubated values [27–30]. Therefore, we have found no evidence to support adding pressure support to “reduce imposed work of breathing” during extubation readiness tests in children, regardless of the endotracheal tube size. It is not like breathing through a straw.
Extubation failure rates are generally low in children, on average 8 % [31], with nearly half from post-extubation UAO [20, 21, 31], which may be difficult to predict during extubation readiness tests [32–34]. With less than 5 % of patients failing extubation from causes other than UAO, it is difficult to demonstrate that one mode of extubation readiness test results in lower reintubation rates. This low extubation failure rate has perhaps lulled pediatric practitioners into a sense of security about using pressure support. If less than 5 % of patients fail extubation when pressure support is used, why should we mandate extubation readiness tests be done on CPAP?
Perhaps our extubation failure rates are too low. This is supported by the greater than 50 % success rate of unplanned extubations in pediatrics [31]; this highlights that many patients can be extubated well before we recognize they are ready. Routine daily spontaneous breathing trials have been advocated, but less than 25 % of pediatric practitioners use them [4]. Perhaps patients can be successfully extubated sooner [2, 35] if we routinely perform spontaneous breathing trials earlier in the mechanical ventilation course [8]. Once we start doing this, on the basis of the physiologic data presented in this paper, it is possible we would have higher extubation failure rates if effort of breathing on pressure support is used during extubation readiness tests. Detecting differences in clinical outcomes such as extubation failure between extubation readiness tests on CPAP versus pressure support would only be feasible if done earlier in the mechanical ventilation course.
From our data, we believe that CPAP of 5 cmH2O alone is sufficient for extubation readiness tests in most children. Our standard practice is a 2-h extubation readiness test on CPAP of 5 cmH2O. While our previous work did not find major differences in effort of breathing between CPAP and T-piece [18], some patients may warrant further reductions to T-piece ventilation (although this may not be advisable in young patients who have active control of end-expiratory lung volume) or warrant longer extubation readiness tests (>2 h), such as those with neuromuscular disease. In addition, some patients warrant CPAP of greater than 5 cmH2O during extubation readiness tests, such as children with obesity. When extubated, these children may use other mechanisms to maintain normal transpulmonary pressures at end exhalation, and weaning to CPAP of 5 cmH2O may result in more alveolar collapse then they would have extubated. Ultimately, the duration of the extubation readiness test and level of end expiratory pressure (CPAP or T-piece) should be determined by an experienced provider mindful of the expected pathophysiology of the patient. However, we believe our data support that pressure support should not be added to this end expiratory pressure to overcome imposed work of breathing, even when extubation is planned to high flow nasal cannula, as CPAP prior to extubation still best estimates post-extubation effort. Because of the limited number of patients on noninvasive CPAP or bi-level positive pressure post-extubation, it is unclear if the same holds when extubating to these modes of noninvasive positive pressure.
Our data also highlight that even on the lowest level of ventilator support to which most patients are weaned (pressure support), effort of breathing is well below normal levels when extubated, corroborating previous investigations [36]. As such, we may be providing too much ventilator support for our patients, which contributes to ventilator-induced diaphragm dysfunction [37], further impairs ventilator weaning, and leads to failed extubation [38, 39].
There are several limitations to our study. First, it is single institution, although we believe generalizability is high given this is a physiologic study. Second, we obtained a convenience sample because we did not study patients on nights or weekends, or patients who did not consent. This may introduce a selection bias. Moreover, these patients were intubated for many reasons (cardiac surgery, pulmonary disease, etc.) and independent of work of breathing, extubation readiness tests may use different criteria based on patient factors. Third, we excluded patients with post-extubation UAO using an “objective” UAO parameter we have previously reported, but there still may be some imprecision in labelling patients with UAO. Fourth, we had only three patients with 2.5-mmID endotracheal tubes, precluding subgroup analysis. Our findings may not generalize to children with 2.5-mmID endotracheal tubes. Fifth, because of institutional practice, noninvasive respiratory support was mostly high flow nasal cannula, and the results may differ with mask CPAP or bi-level positive pressure. Sixth, our illustrative calculations of airway resistance were based on measured patient flow with bench models of endotracheal tubes to determine resistance. Actual resistance in vivo could be lower because of less heat loss when connected to the patient versus exposed to atmosphere or higher if the internal diameter of the endotracheal tube is reduced from secretions or biofilm. Unfortunately we did not measure resistance as part of the study, and it is difficult to attribute measured resistances in vivo to just the upper airway. To that end, there are few published norms for upper airway resistance in spontaneously breathing infants and children, and there are differences between peak resistance (used in our study) and mean resistance. This makes precise definitions of normal values of upper airway resistance complicated [27, 28, 30]. Nevertheless, this will not influence the effort of breathing results. Seventh, the order of pressure support and CPAP was not randomized. Finally, this study did not examine reintubation or extubation failure because of the relatively few cases of reintubation from causes other than UAO.
In conclusion, pressure support should not be added to CPAP to overcome “imposed work of breathing” from the endotracheal tube during spontaneous breathing trials or extubation readiness tests in children. Regardless of the size of the endotracheal tube, the use of pressure support significantly underestimates post-extubation effort of breathing (125–150 % underestimation).
References
Curley MA, Wypij D, Watson RS, Grant MJ, Asaro LS, Cheifetz IM, Dodson BL, Franck LS, Gedeit RG, Angus DC, Matthay MA, RESTORE, PALISI Network (2015) Protocolized sedation vs. usual care in pediatric patients mechanically ventilated for acute respiratory failure. JAMA 313:379–389
Randolph AG, Wypij D, Venkataraman ST, Hanson JH, Gedeit RG, Meert KL, Luckett PM, Forbes P, Lilley M, Thompson J, Cheifetz IM, Hibberd P, Wetzel R, Cox PN, Arnold JH, PALISI Network (2002) Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: a randomized controlled trial. JAMA 288:2561–2568
Fiastro JF, Habib MP, Quan SF (1988) Pressure support compensation for inspiratory work due to endotracheal tubes and demand continuous positive airway pressure. Chest 93:499–505
Mhanna MJ, Anderson IM, Iyer NP, Baumann A (2014) The use of extubation readiness parameters: a survey of pediatric critical care physicians. Respir Care 59:334–339
Fujino Y, Uchiyama A, Mashimo T, Nishimura M (2003) Spontaneously breathing lung model comparison of work of breathing between automatic tube compensation and pressure support. Respir Care 48:38–45
Straus C, Louis B, Isabey D, Lemaire F, Harf A, Brochard L (1998) Contribution of the endotracheal tube and the upper airway to breathing workload. Am J Respir Crit Care Med 157:23–30
Wilson AM, Gray DM, Thomas JG (2009) Increases in endotracheal tube resistance are unpredictable relative to duration of intubation. Chest 136:1006–1013
Newth CJ, Venkataraman S, Willson DF, Meert KL, Harrison R, Dean JM, Pollack M, Zimmerman J, Anand KJ, Carcillo JA, Nicholson CE, Collaborative Pediatric Critical Care Research Network (2009) Weaning and extubation readiness in pediatric patients. Pediatr Crit Care Med 10:1–11
Manczur T, Greenough A, Nicholson GP, Rafferty GF (2000) Resistance of pediatric and neonatal endotracheal tubes: influence of flow rate, size, and shape. Crit Care Med 28:1595–1598
Kolatat T, Aunganon K, Yosthiem P (2002) Airway complications in neonates who received mechanical ventilation. J Med Assoc Thai 85(Suppl 2):S455–S462
Oto J, Imanaka H, Nakataki E, Ono R, Nishimura M (2012) Potential inadequacy of automatic tube compensation to decrease inspiratory work load after at least 48 hours of endotracheal tube use in the clinical setting. Respir Care 57:697–703
Fabry B, Haberthür C, Zappe D, Guttmann J, Kuhlen R, Stocker R (1997) Breathing pattern and additional work of breathing in spontaneously breathing patients with different ventilatory demands during inspiratory pressure support and automatic tube compensation. Intensive Care Med 23:545–552
Haberthür C, Elsasser S, Eberhard L, Stocker R, Guttmann J (2000) Total versus tube-related additional work of breathing in ventilator-dependent patients. Acta Anaesthesiol Scand 44:749–757
Aguilar G, Jover JL, Soro M, Belda FJ, Garcia-Raimundo M, Maruenda A (2005) Additional work of breathing and breathing patterns in spontaneously breathing patients during pressure support ventilation, automatic tube compensation and amplified spontaneous pattern breathing. Eur J Anaesthesiol 22:312–314
Cohen J, Shapiro M, Grozovski E, Fox B, Lev S, Singer P (2009) Prediction of extubation outcome: a randomised, controlled trial with automatic tube compensation vs. pressure support ventilation. Crit Care 13:R21
Cohen JD, Shapiro M, Grozovski E, Lev S, Fisher H, Singer P (2006) Extubation outcome following a spontaneous breathing trial with automatic tube compensation versus continuous positive airway pressure. Crit Care Med 34:682–686
Haberthür C, Mols G, Elsasser S, Bingisser R, Stocker R, Guttmann J (2002) Extubation after breathing trials with automatic tube compensation, T-tube, or pressure support ventilation. Acta Anaesthesiol Scand 46:973–979
Willis BC, Graham AS, Yoon E, Wetzel RC, Newth CJL (2005) Pressure-rate products and phase angles in children on minimal support ventilation and after extubation. Intensive Care Med 31:1700–1705
Khemani R, Flink R, Morzov R, Ross P, Newth C (2013) It’s not like breathing through a straw: effort of breathing on CPAP most accurately estimates post-extubation effort in children. Am J Resp Crit Care Med Suppl 2013.187.1:A3682
Khemani RG, Hotz J, Morzov R, Flink R, Kamerkar A, Ross PA, Newth CJ (2016) Evaluating risk factors for pediatric post-extubation upper airway obstruction using a physiology-based tool. Am J Respir Crit Care Med 193:198–209
Khemani R, Flink R, Hotz J, Ross P, Ghuman A, Newth CJ (2015) Respiratory inductance plethysmography calibration for pediatric upper airway obstruction: an animal model. Pediatr Res 77:75–83
Sackner MA, Watson H, Belsito AS, Feinerman D, Suarez M, Gonzalez G, Bizousky F, Krieger B (1989) Calibration of respiratory inductive plethysmograph during natural breathing. J Appl Physiol 66:410–420
Ross PA, Hammer J, Khemani R, Klein M, Newth CJ (2010) Pressure-rate product and phase angle as measures of acute inspiratory upper airway obstruction in rhesus monkeys. Pediatr Pulmonol 45:639–644
Diblasi RM, Zignego JC, Tang DM, Hildebrandt J, Smith CV, Hansen TN, Richardson CP (2010) Noninvasive respiratory support of juvenile rabbits by high-amplitude bubble continuous positive airway pressure. Pediatr Res 67:624–629
Argent AC, Hatherill M, Newth CJ, Klein M (2008) The effect of epinephrine by nebulization on measures of airway obstruction in patients with acute severe croup. Intensive Care Med 34:138–147
Argent AC, Newth CJ, Klein M (2008) The mechanics of breathing in children with acute severe croup. Intensive Care Med 34:324–332
Davis S, Gappa M, Rosenfeld M (2005) Respiratory mechanics. In: Hammer J, Eber E (eds) Paediatric pulmonary function testing. Karger, Basel, p 30
Masters IB, Seidenberg J, Hudson I, Phelan PD, Olinsky A (1987) Longitudinal study of lung mechanics in normal infants. Pediatr Pulmonol 3:3–7
Hammer J, Patel N, Newth CJ (2003) Effect of forced deflation maneuvers upon measurements of respiratory mechanics in ventilated infants. Intensive Care Med 29:2004–2008
Hammer J, Numa A, Newth CJ (1997) Acute respiratory distress syndrome caused by respiratory syncytial virus. Pediatr Pulmonol 23:176–183
Kurachek SC, Newth CJ, Quasney MW, Rice T, Sachdeva RC, Patel NR, Takano J, Easterling L, Scanlon M, Musa N, Brilli RJ, Wells D, Park GS, Penfil S, Bysani KG, Nares MA, Lowrie L, Billow M, Chiochetti E, Lindgren B (2003) Extubation failure in pediatric intensive care: a multiple-center study of risk factors and outcomes. Crit Care Med 31:2657–2664
Wratney AT, Benjamin DK Jr, Slonim AD, He J, Hamel DS, Cheifetz IM (2008) The endotracheal tube air leak test does not predict extubation outcome in critically ill pediatric patients. Pediatr Crit Care Med 9:490–496
Mhanna MJ, Zamel YB, Tichy CM, Super DM (2002) The “air leak” test around the endotracheal tube, as a predictor of postextubation stridor, is age dependent in children. Crit Care Med 30:2639–2643
Khemani R, Hotz J, Morzov R, Kamerkar A, Flink R, PA R, Newth CJ (2015) Risk factors for pediatric post-extubation upper airway obstruction. Am J Respir Crit Care Med 191:A4068
Jouvet PA, Payen V, Gauvin F, Emeriaud G, Lacroix J (2013) Weaning children from mechanical ventilation with a computer-driven protocol: a pilot trial. Intensive Care Med 39:919–925
Emeriaud G, Larouche A, Ducharme-Crevier L, Massicotte E, Flechelles O, Pellerin-Leblanc AA, Morneau S, Beck J, Jouvet P (2014) Evolution of inspiratory diaphragm activity in children over the course of the PICU stay. Intensive Care Med 40:1718–1726
Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, Rittayamai N, Lanys A, Tomlinson G, Singh JM, Bolz SS, Rubenfeld GD, Kavanagh BP, Brochard LJ, Ferguson ND (2015) Evolution of diaphragm thickness during mechanical ventilation. Impact of inspiratory effort. Am J Respir Crit Care Med 192:1080–1088
Wolf GK, Walsh BK, Green ML, Arnold JH (2011) Electrical activity of the diaphragm during extubation readiness testing in critically ill children. Pediatr Crit Care Med 12:e220–224
DiNino E, Gartman EJ, Sethi JM, McCool FD (2014) Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax 69:423–427
Acknowledgments
The authors would like to thank Aaron Clute, Ed Guerrero, Edwin Khatchetourian, and Cary Sodetani RCPs for their assistance with the study protocol; Anoopindar Bhalla, Sarah Rubin, and Timothy Deakers for their assistance with consents; Jeffery Terry and Paul Vee for administrative support, and all the bedside providers in the Children’s Hospital Los Angeles pediatric intensive care unit and cardiothoracic intensive care unit for their participation and support. Sources of support National Institutes of Health/NICHD 1K23HL103785, Los Angeles Basin Clinical Translational Science Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Work performed at the Children’s Hospital Los Angeles.
Take-home message: By performing direct measures of patient effort of breathing before and after extubation on over 400 mechanically ventilated children, we have found that patient effort of breathing on CPAP of 5 cmH2O alone provides a good estimate of post-extubation effort. Regardless of the endotracheal tube size, pressure support results in significant under estimation of post-extubation effort of breathing.
Rights and permissions
About this article
Cite this article
Khemani, R.G., Hotz, J., Morzov, R. et al. Pediatric extubation readiness tests should not use pressure support. Intensive Care Med 42, 1214–1222 (2016). https://doi.org/10.1007/s00134-016-4387-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4387-3