Abstract
Purpose
The soluble form of the receptor for advanced glycation end-products (sRAGE) is a promising marker for epithelial dysfunction, but it has not been fully characterized as a biomarker of acute respiratory distress syndrome (ARDS). Whether sRAGE could inform on the response to ventilator settings has been poorly investigated, and whether a recruitment maneuver (RM) may influence plasma sRAGE remains unknown.
Methods
Twenty-four patients with moderate/severe, nonfocal ARDS were enrolled in this prospective monocentric crossover study and randomized into a “RM-SHAM” group when a 6-h-long RM sequence preceded a 6-h-long sham evaluation period, or a “SHAM-RM” group (inverted sequences). Protective ventilation was applied, and RM consisted of the application of 40 cmH2O airway pressure for 40 s. Arterial blood was sampled for gas analyses and sRAGE measurements, 5 min pre-RM (or 40-s-long sham period), 5, 30 min, 1, 4, and 6 h after the RM (or 40-s-long sham period).
Results
Mean PaO2/FiO2, tidal volume, PEEP, and plateau pressure were 125 mmHg, 6.8 ml/kg (ideal body weight), and 13 and 26 cmH2O, respectively. Median baseline plasma sRAGE levels were 3,232 pg/ml. RM induced a significant decrease in sRAGE (−1,598 ± 859 pg/ml) in 1 h (p = 0.043). At 4 and 6 h post-RM, sRAGE levels increased back toward baseline values. Pre-RM sRAGE was associated with RM-induced oxygenation improvement (AUC 0.84).
Conclusions
We report the first kinetics study of plasma sRAGE after RM in ARDS. Our findings reinforce the value of plasma sRAGE as a biomarker of ARDS.
Similar content being viewed by others
Introduction
Acute respiratory distress syndrome (ARDS), a major cause of respiratory failure and death in critically ill patients [1], is characterized by diffuse alveolar epithelial and lung endothelial injury leading to increased permeability pulmonary edema [2, 3]. Despite recent therapeutic advances [4–6], neither effective pharmacologic therapies nor fully characterized biomarkers have yet been identified [7–9].
A biomarker should have pathophysiological significance, provide a diagnosis, assess disease severity or risk, and guide clinical interventions [10, 11]. The receptor for advanced glycation end-products (RAGE), a member of the immunoglobulin superfamily, is a transmembrane receptor that can bind multiple ligands, e.g., advanced glycation end-products, high-mobility group box-1, and S100 proteins. RAGE–ligand interaction results in intracellular signaling, which leads to activation of the proinflammatory transcription factor nuclear factor-κB (NF-κB) [12]. This cellular activation is related to inflammatory processes or tissue injury, and RAGE is now recognized as a marker of alveolar type (AT) I cell injury [13, 14]. Elevated sRAGE levels during ARDS are not influenced by associated sepsis but correlate with diffuse CT scan damage and mortality in patients ventilated with high tidal volumes [15], and sRAGE levels are reported to decrease when PaO2/FiO2 increases during the resolution of ARDS [16]. The Berlin definition of ARDS does not include any specifications about the distribution of bilateral infiltrates [3]; however, the expert panel suggests that this area may deserve additional investigation [3]. In this perspective, measuring sRAGE might help to identify subgroups of ARDS patients with poor clinical outcomes [15, 16].
Whether sRAGE could help to tailor therapy or to predict response to therapy (e.g., ventilator settings) in patients with ARDS remains unknown, and few data are available to date on the influence of ventilator settings on plasma sRAGE during ARDS. In a retrospective analysis of patients enrolled in a randomized controlled trial [15], baseline plasma levels of sRAGE were associated with mortality in those receiving high tidal volumes [12 ml/kg ideal body weight (IBW)], supporting the hypothesis of a protective effect of lower volumes. As part of a lung-protective ventilation strategy, recruitment maneuvers (RM) can be applied to increase arterial oxygenation, net alveolar fluid clearance (AFC), and alveolar recruitment [17–20]. Lung morphology, as assessed by loss of aeration distribution patterns on CT scan, predicts the response to RM in ARDS, and patients presenting with nonfocal morphology are more likely to respond to RM [21]. To date, the kinetics of plasma sRAGE levels after RM have never been investigated in the clinical setting [22], and whether sRAGE may serve as a marker of the short-term response to ventilator settings (e.g., by predicting the response to RM with regard to oxygenation improvement after RM) remains unexplored.
We hypothesized that RM would result in decreased plasma sRAGE in ARDS. Our study was therefore designed to determine short-term effects of RM on plasma sRAGE in patients with diffuse ARDS, and to test sRAGE as a predictor of response to RM.
Partial results of this study were presented as an oral communication during the conference “Congrès National de la Société de Réanimation de Langue Française” (2014) [23] and during the annual congress of the European Society of Intensive Care Medicine (2014) [24].
Materials and methods
Setting
The protocol for this monocenter, single-blind, crossover randomized controlled trial was approved by our institutional review board (Comité de Protection des Personnes Sud Est VI, AU948). Next-of-kin written consent was obtained for all participants, followed whenever possible by patient consent as soon as they regained the capacity to provide it. There was no deviation from the approved protocol.
Patients
Patients were eligible if they were over 18 years old and admitted to the ICU within 24 h of moderate or severe ARDS onset, according to the Berlin definition [3]. ARDS criteria had to be simultaneously met within 1 week of a known clinical insult [3]. Only patients with diffuse (nonfocal) lung CT scan morphology were enrolled. Two independent radiologists performed the qualitative CT analysis according to the “CT scan ARDS study group” criteria [25–27]. Patterns of loss of aeration distribution were characterized, and nonfocal pattern was identified with diffuse or patchy loss of aeration [17].
Patients were ineligible if they had a history of allergy to cisatracurium, acute complications of diabetes due to severe hyperglycemia (diabetic ketoacidosis or hyperosmolar hyperglycemic nonketotic syndrome), dialysis for end-stage kidney disease, Alzheimer’s disease, amyloidosis, evolutive solid neoplasm; intracranial hypertension, bronchopleural fistula, or pneumothorax was suspected or confirmed; they received long-term oxygen therapy or respiratory support for chronic respiratory disease; they had already received RM since ARDS diagnosis. Only patients with stabilizing hemodynamic status (defined as the absence of preload dependence and a recent trend for decreasing blood lactate levels and norepinephrine requirements) were eligible.
Interventions
Patients were assigned into two groups: a “RM-SHAM” group when an RM sequence preceded a sham evaluation period, and a “SHAM-RM” group, in which patients received a sham sequence first; each sequence was 6-h long and included a 2-h wash-out period (Fig. 1).
RM consisted of the application of 40 cmH2O continuous positive airway pressure for 40 s [19] in patients under deep sedation and neuromuscular blockade by cisatracurium (intravenous bolus of 0.15 mg/kg followed by a continuous infusion of 37.5 mg/h, in both “RM-SHAM” and “SHAM-RM” groups).
All patients received lung-protective ventilation (see Electronic Supplementary Material). Fraction of inspired oxygen and positive end-expiratory pressure (PEEP) were maintained unchanged during the experimental period. Apart from the experimental procedures, intensive care management was conducted using standard protocols; weaning from mechanical ventilation, sepsis management, and the use of sedative agents were based on available guidelines [4, 28, 29].
Biologic sample collection and measurements
During both sequences, arterial blood was sampled from an indwelling catheter, 5 min before RM (or a 40-s-long sham period) and 5, 30 min, 1, 4, and 6 h after RM (or a 40-s-long sham period). Blood gases were immediately analyzed after sample collection. Other samples were centrifuged at 1,000×g for 15 min; supernatant was kept frozen at −80 °C until analysis. Plasma levels of sRAGE were measured in duplicate using commercially available ELISA kits (R&D Systems, Minneapolis, MN). The personnel responsible for performing sRAGE assays had no knowledge of the clinical data and of the randomization group.
Study outcomes
The primary outcome was plasma levels of sRAGE, as measured 1 h after RM. Secondary outcomes included the kinetics of plasma sRAGE during the first 6 h after RM and the predictive value of baseline sRAGE on the response to RM (as defined, a priori, as a 20 % increase in PaO2 1 h after RM [17]).
Statistical analysis
All analyses were performed using Stata software (v13, StataCorp, College Station, TX). Qualitative data are expressed as numbers and percentages, and quantitative data as mean, standard deviation (SD) (or SEM for sRAGE for graph readability) or median and interquartile range (IQR). To compare baseline characteristics between groups, Student’s t test or the Mann–Whitney test was considered for quantitative parameters according to t test hypotheses (normality assumption using the Shapiro–Wilk test and homoscedasticity with the Fisher–Snedecor test). Proportions were compared among groups using the Chi square test or Fisher’s exact test. Considering a crossover design and repeated correlated data, a complete random-effects model was used to study the longitudinal evolution of sRAGE (aim analysis): (1) taking into account between- and within-subject variability (random subject effects: random intercept and slope), and (2) evaluating fixed effects: group, time-points evaluation, and their interaction, period, treatment, sequence, and possible carryover. Residual normality was checked for all models. This analysis was completed by standard crossover statistical analyses with the pkcross routine in Stata. The default parameterization estimates overall mean, period effects, treatment effects, and sequence effects, assuming possible carryover effects. As sRAGE was not normally distributed, values were log-transformed in order to achieve normality and to allow the correct use of the statistical approach. Receiver-operating characteristic (ROC) curve was computed and area under the curve was used to evaluate how well the model distinguished non-response from response to RM. Confidence intervals (CIs) for areas under ROC curve were calculated using non-parametric assumptions. Several indexes were calculated (Youden, Liu, and efficiency) to propose the threshold value that optimized the sensitivity and the specificity of sRAGE to predict the response. The study of relations between quantitative parameters (sRAGE with outcomes) was improved by calculating correlation coefficients (Pearson or Spearman according to statistical distribution); p < 0.05 (two-sided) was considered statistically significant.
Only few data are available on the variability of sRAGE levels in critically ill patients [14–16], with standard deviations around 2,000 pg/ml. When considering alpha and beta risks of 5 % (bilateral) and 20 %, respectively, enrolling 12 patients in each group would allow the detection of a 1,700 pg/ml difference in sRAGE levels (measured 1 h after RM) between groups for a null correlation coefficient, a 1,200 pg/ml difference for a 0.5 coefficient, and a 2,400 pg/ml difference at the end of the crossover period. If an interaction effect of the first period over the second one was observed, only results from the first period were considered.
Results
Study population
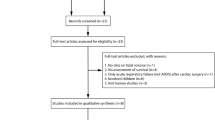
From June 2012 through October 2013, 35 patients with moderate to severe ARDS were assessed for eligibility, and 24 were included (Fig. 2). Data on the primary outcome were available for all. Pneumonia was the most common cause of ARDS. First plasma samples for the study were drawn a median of 1 [IQR 1, 2] day after ICU admission and a median of 18 [10, 22] h after intubation.
At baseline in the whole cohort (N = 24), mean (±SD) PaO2/FiO2 was 125 ± 38 mmHg, mean tidal volumes were 6.8 ± 0.9 ml/kg IBW, PEEP was 13.4 ± 3 cmH2O and plateau pressure was 26 ± 4 cmH2O. Baseline characteristics are reported in Table 1. No difference was found in baseline plasma sRAGE between patients receiving and those not receiving cisatracurium prior to randomization [3,597 (2,718, 4,501) pg/ml versus 3,636 (2,941, 10,070), respectively, p = 0.55]. There was no difference in baseline plasma sRAGE between patients receiving and those not receiving corticosteroids at randomization [3,603 (3,005, 6,431) versus 3,583 (2,715, 4,837) pg/ml, respectively, p = 0.96].
Kinetics of plasma sRAGE after RM
Baseline plasma sRAGE levels, as measured 5 min before RM or sham period depending on treatment order, were 3,551 [2,866, 4,765] and 3,203 [2,650, 3,661], respectively (p = 0.15).
Longitudinal data analysis based on a mixed statistical model confirmed a significant decrease in plasma sRAGE 1 h after RM [−1,545 (−1,931, −1,029), p = 0.049] (Fig. 3). Figure 3 summarizes the evolution of mean plasma sRAGE levels during both sequences; all measured sRAGE concentrations are reported in Table 2 (Electronic Supplementary Material). A “time” effect was found in the evolution of sRAGE levels (p = 0.001): the decrease in sRAGE was more pronounced when RM was applied during the first sequence, but the decrease after RM was significant in both first (p = 0.015) and second (p = 0.01) sequences.
Oxygenation and respiratory parameters and their variation in both sequences are summarized in Table 3 (Electronic Supplementary Material); no difference was found between the two sequences, apart from lower inspiratory plateau pressure 5 min after RM as compared with sham sequence. No change in mean arterial pressure or in norepinephrine dose over time was found to be significant in our study (p = 0.11 and 0.9, respectively),
Baseline plasma sRAGE and response to RM
A total of eight patients responded to RM, i.e., had a 20 % improvement in PaO2 1 h after RM (Fig. 4a). The area under the ROC when baseline plasma sRAGE was used to differentiate response from non-response to RM was 0.84 (95 % confidence interval 0.66–1) (Fig. 4b). A cut-off value of 4,501 pg/ml had a sensitivity of 83 % (95 % CI 36–99) and a specificity of 83 % (95 % CI 59–96).
a Individual values of plasma sRAGE (pg/ml), as measured before and 1 h after the recruitment maneuver (RM). b Receiver-operating characteristic curve of baseline plasma sRAGE levels in differentiating between the response and the absence of response to a recruitment maneuver. The area under the receiver-operating characteristic curve was 0.84 (95 % CI 0.66–1) for a cut-off value of 4,501 pg/ml, with a sensitivity of 83 % (95 % CI 36–99) and a specificity of 83 % (95 % CI 59–96)
Patient outcome
No significant correlation was found between plasma sRAGE as measured at baseline and main patient outcomes, except between baseline sRAGE (but not post-RM sRAGE) and the number of ventilator-free days at day 28 (Spearman’s rank correlation coefficient ρ = 0.4; p = 0.03) (Table 4, Electronic Supplementary Material). Correlations between patient outcome and “delta” sRAGE (defined as the difference between plasma sRAGE as measured 1 h after RM and plasma sRAGE as measured before RM) were not statistically significant (Table 4, Electronic Supplementary Material).
Discussion
We report here the kinetics of RM-induced changes in plasma sRAGE, a marker of AT I cell injury [13, 14]. A significant but transient decrease in sRAGE was observed in patients with diffuse ARDS, and baseline sRAGE was associated with response to RM.
In our study, RM has a significant biological impact, with rapid and transient decrease in plasma sRAGE. Previous studies raised the question of RM-induced exacerbation of epithelial [30, 31] and endothelial [32] injury, increasing alveolar-capillary permeability in non-responders [17]. Furthermore, if RM is applied to an inhomogeneous lung parenchyma, its deleterious effects may be amplified, predisposing alveolar cells to deformation during lung distension and causing nonphysiological stress and strain [33]. Our results could be in contradiction with previous data [30, 31], but whether RM-induced fall in sRAGE could reflect a beneficial epithelial effect of RM remains uncertain, and our findings warrant further investigation.
Our measurements support the hypothesis of a rapid but transient decrease in sRAGE release from the alveolar structure to the systemic circulation after RM. The extrapolation of such findings to pathophysiological hypotheses remains hazardous. Multiple mechanisms seem implicated in sRAGE expression but they remain underinvestigated to date. In a recent experimental study in a rat model of endotoxin-induced lung injury, higher RAGE mRNA levels were rapidly induced after RM as compared to controls [22]. These results are not incompatible with ours. As a matter of fact, RAGE mRNA participates in the expression of full-length RAGE but sRAGE expression implicates additional complex regulation by proteases [34]. Such a regulation could be influenced by the accumulation of various RAGE ligands and oxidative stress [34–37], resulting in high expression of full-length RAGE. Thus, less cleavage of full-length RAGE by proteases could explain, at least in part, a decrease in sRAGE levels in our study. Unfortunately, the respective effects on plasma sRAGE of the type, the abruptness, and the duration of the RM were beyond the scope of our study [22].
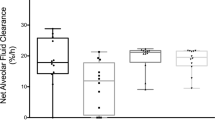
The response to RM was defined a priori by an increase in PaO2 as measured 1 h after RM [17], and a cut-off value of 4,501 pg/ml predicted response to RM with good sensitivity and specificity. This finding is compatible with previous results, in particular with a cut-off value of plasma sRAGE that could distinguish between nonfocal and diffuse lung morphology in ARDS [16]. In our study, few patients responded to RM, i.e., improved their oxygenation with RM, but a decrease in plasma sRAGE was observed almost in all (23 out of 24 patients) (Fig. 4a). In an ex vivo model of isolated perfused human lung, alveolar sRAGE was inversely associated with AFC rate [38]. If sRAGE reflects AFC, then the effect of RM on sRAGE could provide indirect information on AFC, even in cases of unchanged PaO2/FiO2 [19]. The measurable oxygenation benefit of RM may be mainly dependent on the pressure level required to reverse atelectasis [19]. An RM-induced increase in regional AFC may simply mean that regional alveolar edema is reduced as fluid is “pushed out” from the alveoli during RM application. If RM pressure level is not high enough to simultaneously reverse atelectasis, the resulting change in oxygenation may be minor. However, there would still be a regional, RM-associated benefit with respect to AFC, which may be measurable through the change in sRAGE.
Our study has limitations. First, this was a relatively small, single-center study, partly due to protocol complexity. Second, as there was no change in PEEP levels during the whole study period, and because few data only are available to date in the existing literature [15], how PEEP levels influence plasma sRAGE remains to be investigated. In addition, whether this response is associated or not with effective alveolar recruitment or changes in systemic inflammation was not investigated in our study. Next, although results at baseline are consistent with the absence of a corticosteroid or cisatracurium effect on plasma sRAGE, future studies are needed to investigate this specific issue. Also, we cannot exclude that a transient RM-induced decrease in pulmonary blood flow could result in decreased sRAGE release from the lung. Finally, AFC evaluation relies on edema fluid aspirates with assessment of differences in total protein levels over time [17, 39]. As this measurement would have hampered the expected beneficial effects of RM by causing alveolar de-recruitment, it was not assessed in our study.
Our findings add to the growing body of evidence supporting sRAGE as a biomarker during ARDS and represent one more step toward better characterizing sRAGE as a tool to tailor ventilation in ARDS [21]. Whether monitoring plasma sRAGE could be beneficial in the management of ARDS patients by reflecting lung response to therapy (e.g., ventilator strategy) remains unknown, but is currently under investigation in a large randomized controlled trial of lung imaging for ventilator setting in ARDS [40].
In conclusion, we report the first kinetics study of plasma levels of sRAGE after RM in ARDS. Plasma sRAGE decreased significantly 1 h after RM before evolving toward its baseline levels between 4 and 6 h, and baseline sRAGE was associated with response to RM, i.e., improved oxygenation. Our findings reinforce the value of plasma sRAGE as a biomarker of ARDS, and they may stimulate more research on the assessment and validation of sRAGE as a surrogate marker of response to clinical interventions during ARDS.
References
Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, Bion J, Romand JA, Villar J, Thorsteinsson A, Damas P, Armaganidis A, Lemaire F (2004) Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med 30:51–61
Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. N Engl J Med 342:1334–1349
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS (2012) Acute respiratory distress syndrome: the Berlin definition. JAMA 307:2526–2533
The Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, Constantin JM, Courant P, Lefrant JY, Guerin C, Prat G, Morange S, Roch A (2010) Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 363:1107–1116
Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L (2013) Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 368:2159–2168
Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA Jr, Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL (2003) Future research directions in acute lung injury: summary of a national heart, lung, and blood institute working group. Am J Respir Crit Care Med 167:1027–1035
Ware LB, Koyama T, Billheimer DD, Wu W, Bernard GR, Thompson BT, Brower RG, Standiford TJ, Martin TR, Matthay MA (2010) Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest 137:288–296
Curley GF, Laffey JG (2015) Future therapies for ARDS. Intensive Care Med 41:322–326
Ray P, Le Manach Y, Riou B, Houle TT (2010) Statistical evaluation of a biomarker. Anesthesiology 112:1023–1040
Terpstra ML, Aman J, van Nieuw Amerongen GP, Groeneveld AB (2014) Plasma biomarkers for acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med 42:691–700
Schmidt AM, Yan SD, Yan SF, Stern DM (2001) The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest 108:949–955
Shirasawa M, Naoyuki F, Susumu H, Hideki O, Junko I, Koshi M, Yutaka H (2004) Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes Cells 9:165–174
Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA (2006) Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med 173:1008–1015
Calfee CS, Ware LB, Eisner MD, Parsons PE, Thompson BT, Wickersham N, Matthay MA (2008) Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax 63:1083–1089
Jabaudon M, Futier E, Roszyk L, Chalus E, Guerin R, Petit A, Mrozek S, Perbet S, Cayot-Constantin S, Chartier C, Sapin V, Bazin JE, Constantin JM (2011) Soluble form of the receptor for advanced glycation end products is a marker of acute lung injury but not of severe sepsis in critically ill patients. Crit Care Med 39:480–488
Constantin JM, Cayot-Constantin S, Roszyk L, Futier E, Sapin V, Dastugue B, Bazin JE, Rouby JJ (2007) Response to recruitment maneuver influences net alveolar fluid clearance in acute respiratory distress syndrome. Anesthesiology 106:944–951
Constantin JM, Futier E, Cherprenet AL, Chanques G, Guerin R, Cayot-Constantin S, Jabaudon M, Perbet S, Chartier C, Jung B, Guelon D, Jaber S, Bazin JE (2010) A recruitment maneuver increases oxygenation after intubation of hypoxemic intensive care unit patients: a randomized controlled study. Crit Care 14:R76
Constantin JM, Jaber S, Futier E, Cayot-Constantin S, Verny-Pic M, Jung B, Bailly A, Guerin R, Bazin JE (2008) Respiratory effects of different recruitment maneuvers in acute respiratory distress syndrome. Crit Care 12:R50
Suzumura EA, Figueiro M, Normilio-Silva K, Laranjeira L, Oliveira C, Buehler AM, Bugano D, Passos Amato MB, Ribeiro Carvalho CR, Berwanger O, Cavalcanti AB (2014) Effects of alveolar recruitment maneuvers on clinical outcomes in patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Intensive Care Med 40:1227–1240
Constantin JM, Grasso S, Chanques G, Aufort S, Futier E, Sebbane M, Jung B, Gallix B, Bazin JE, Rouby JJ, Jaber S (2010) Lung morphology predicts response to recruitment maneuver in patients with acute respiratory distress syndrome. Crit Care Med 38:1108–1117
Silva PL, Moraes L, Santos RS, Samary C, Ramos MB, Santos CL, Morales MM, Capelozzi VL, Garcia CS, de Abreu MG, Pelosi P, Marini JJ, Rocco PR (2013) Recruitment maneuvers modulate epithelial and endothelial cell response according to acute lung injury etiology. Crit Care Med 41:e256–e265
Jabaudon M, Hamroun N, Roszyk L, Guérin R, Perbet S, Pascal J, Cayot S, Futier E, Sapin V, Constantin JM (2013) Effets d’une manœuvre de recrutement alvéolaire sur les concentrations plasmatiques de la forme soluble du récepteur des produits de glycation avancée (sRAGE) dans les formes diffuses de syndrome de détresse respiratoire aiguë [abstract]. Réanimation 23:S107–S111
Jabaudon M, Hamroun N, Roszyk L, Blondonnet R, Guerin R, Perbet S, Pascal J, Cayot S, Futier E, Sapin V, Constantin JM (2014) Effects of a recruitment maneuver on plasma levels of sRAGE, the soluble form of the receptor for advanced glycation end products, in patients with diffuse acute respiratory distress syndrome (ARDS) [abstract]. Intensive Care Med 40(Suppl 1):0855
Puybasset L, Cluzel P, Gusman P, Grenier P, Preteux F, Rouby JJ, CT Scan ARDS Study Group (2000) Regional distribution of gas and tissue in acute respiratory distress syndrome. I. Consequences for lung morphology. Intensive Care Med 26:857–869
Rouby JJ, Puybasset L, Cluzel P, Richecoeur J, Lu Q, Grenier P, CT Scan ARDS Study Group (2000) Regional distribution of gas and tissue in acute respiratory distress syndrome. II. Physiological correlations and definition of an ARDS Severity Score. Intensive Care Med 26:1046–1056
Puybasset L, Gusman P, Muller JC, Cluzel P, Coriat P, Rouby JJ, CT Scan ARDS Study Group (2000) Regional distribution of gas and tissue in acute respiratory distress syndrome. III. Consequences for the effects of positive end-expiratory pressure. Intensive Care Med 26:1215–1227
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R (2013) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39:165–228
Antonelli M, Bonten M, Chastre J, Citerio G, Conti G, Curtis JR, De Backer D, Hedenstierna G, Joannidis M, Macrae D, Mancebo J, Maggiore SM, Mebazaa A, Preiser JC, Rocco P, Timsit JF, Wernerman J, Zhang H (2012) Year in review in Intensive Care Medicine 2011: III. ARDS and ECMO, weaning, mechanical ventilation, noninvasive ventilation, pediatrics and miscellanea. Intensive Care Med 38:542–556
de Prost N, Dreyfuss D, Saumon G (2007) Evaluation of two-way protein fluxes across the alveolo-capillary membrane by scintigraphy in rats: effect of lung inflation. J Appl Physiol 102:794–802
Silva PL, Moraes L, Santos RS, Samary C, Ornellas DS, Maron-Gutierrez T, Morales MM, Saddy F, Capelozzi VL, Pelosi P, Marini JJ, Gama de Abreu M, Rocco PR (2011) Impact of pressure profile and duration of recruitment maneuvers on morphofunctional and biochemical variables in experimental lung injury. Crit Care Med 39:1074–1081
Silva PL, Cruz FF, Fujisaki LC, Oliveira GP, Samary CS, Ornellas DS, Maron-Gutierrez T, Rocha NN, Goldenberg R, Garcia CS, Morales MM, Capelozzi VL, Gama de Abreu M, Pelosi P, Rocco PR (2010) Hypervolemia induces and potentiates lung damage after recruitment maneuver in a model of sepsis-induced acute lung injury. Crit Care 14:R114
Gattinoni L, Carlesso E, Langer T (2012) Towards ultraprotective mechanical ventilation. Curr Opin Anaesthesiol 25:141–147
Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, Reiss K, Saftig P, Bianchi ME (2008) A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J 22:3716–3727
Schmidt AM, Yan SD, Yan SF, Stern DM (2000) The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta 1498:99–111
Hergrueter AH, Nguyen K, Owen CA (2011) Matrix metalloproteinases: all the RAGE in the acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 300:L512–L515
Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP (2005) Understanding RAGE, the receptor for advanced glycation end products. J Mol Med 83:876–886
Briot R, Frank JA, Uchida T, Lee JW, Calfee CS, Matthay MA (2009) Elevated levels of the receptor for advanced glycation end products, a marker of alveolar epithelial type I cell injury, predict impaired alveolar fluid clearance in isolated perfused human lungs. Chest 135:269–275
Matthay MA, Wiener-Kronish JP (1990) Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis 142:1250–1257
Constantin JM (2014) Lung imaging for ventilatory setting in ARDS. Clinicaltrials.gov registration number: NCT02149589 http://www.clinicaltrials.gov/show/NCT02149589. Accessed 31 Oct 2014
Acknowledgments
The authors thank the nurses and all the staff of the intensive care unit and the laboratories at Estaing Hospital, for patient management and their valuable help in data collection.
Conflicts of interest
This work was supported by grants from the Auvergne Regional Council (“Programme Nouveau Chercheur de la Région Auvergne” 2013), the French Agence Nationale de la Recherche, and the Direction Générale de l’Offre de Soins (“Programme de Recherche Translationnelle en Santé” ANR-13-PRTS-0010). The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take-home message: The authors describe the first kinetics study of plasma sRAGE after a recruitment maneuver in ARDS. These findings support the value of sRAGE as a marker of short-term response to therapeutic interventions and reinforce sRAGE as a biomarker of ARDS.
Trial registration: ClinicalTrials.gov identifier NCT01600651.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jabaudon, M., Hamroun, N., Roszyk, L. et al. Effects of a recruitment maneuver on plasma levels of soluble RAGE in patients with diffuse acute respiratory distress syndrome: a prospective randomized crossover study. Intensive Care Med 41, 846–855 (2015). https://doi.org/10.1007/s00134-015-3726-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-015-3726-0