Abstract
Purpose
Adherence to full sterile procedures may be compromised when central venous catheters are inserted as part of emergency resuscitation and stabilisation, particularly outside the intensive care unit. Half of emergency admissions to paediatric intensive care units (PICU) in the UK occur after stabilisation at other hospitals. We determined whether bloodstream infection (BSI) occurred more frequently in children admitted to PICU after inter-hospital transfer compared to within-hospital admissions.
Methods
Data on emergency admissions to 20 PICUs in England and Wales for children <16 years between 2003–2012 were linked from the national PICU audit database (PICANet) and national infection surveillance (LabBase2). PICU-acquired BSI was defined as any positive blood culture sampled between 2 days after admission and 2 days following discharge from PICU.
Results
A total of 32,861/62,515 (53 %) admissions were inter-hospital transfers. Multivariable regression showed no significant difference in rates of PICU-acquired BSI by source of admission (incidence-rate ratio for inter-hospital transfer versus within-hospital admission = 0.97; 95 % CI 0.87–1.07) after adjusting for other risk-factors. Rates decreased more rapidly between 2003 and 2012 for inter-hospital transfers: 17.0 % (95 % CI 14.9–19.0 % per year) compared with 12.4 % (95 % CI 9.9–14.9 % per year) for within-hospital admissions. The median time to first PICU-acquired BSI did not differ significantly between inter-hospital transfers (7 days; IQR 4–13) and within-hospital admissions (8 days; IQR 4–15).
Conclusions
Nationally, inter-hospital transfer is no longer a significant risk factor for PICU-acquired BSI. Given the large proportion of infection occurring in the second week of admission, initiatives to further reduce PICU-acquired BSI should focus on maintaining sterile procedures after admission.
Similar content being viewed by others
Introduction
Bloodstream infections (BSI) are an important cause of mortality, prolonged stay and excess healthcare costs in paediatric intensive care units (PICU) [1–3]. An estimated 70 % of BSIs occurring in PICU are thought to be related to the use of central venous catheters (CVCs) [4]. Over the past decade, implementation of care bundles to improve the sterility of CVC insertion have resulted in significant reductions in the rate of catheter-related BSI in adult and paediatric ICUs worldwide [5–9].
Risk factors for BSI in critically ill children with a CVC relate to patient susceptibility (e.g. immunodeficiency, need for blood transfusion and renal replacement therapy) and the catheter (sterility of insertion, type of catheter material, number of CVCs and duration of catheterisation) [10–12]. The potential for full aseptic technique during CVC insertion is likely to be compromised during emergency stabilisation. This has resulted in national guidelines recommending elective replacement of the CVC after admission to intensive care [13–17]. Evidence from single-centre studies showing an increased risk of BSI in patients whose CVC was inserted outside the ICU underpins these guidelines, but partly predates major improvements in infection control across the UK National Health Service, including specific bundles of care to improve sterile procedures during CVC insertion [13].
We use national linked data to determine how trends in BSI differed in children admitted after inter-hospital transfer compared with within-hospital admission to PICU. Our study covers a period of 10 years, during which time national quality improvement initiatives related to CVC care were implemented (Saving Lives in 2007 and Matching Michigan in 2009) [18, 19]. Falling rates of BSI over recent years have not been restricted to children with CVCs but have been observed in all children in PICU (who may be subjected to a number of invasive procedures). We therefore chose to include all children admitted to PICU in this study.
Materials and methods
Study population
The study population comprised all admissions between March 2003 and December 2012 of children aged <16 years to the 22 PICUs in England and Wales with at least 200 admissions per year. Data were extracted from the Paediatric Intensive Care Audit Network (PICANet), which records source of admission and clinical information but does not capture information on BSI or CVC insertion [20]. All PICUs in England and Wales have contributed data to PICANet since March 2003.
We linked records of PICU admissions to records of BSI recorded in the national infection surveillance system in England and Wales (LabBase2). This is a voluntary system coordinated by Public Health England (PHE) [21]. Hospital laboratories are requested to report all clinically significant isolates from blood, but there is variation in how clinical significance is defined and hence in judging which isolates should be reported. We have previously reported 80–95 % ascertainment of BSI by Labbase2 and shown that incomplete reporting is not related to patient or organism characteristics [22]. The linked dataset comprised admissions to 20/22 PICUs in England and Wales (two PICUs did not report sufficient data to LabBase2 during the study period).
A detailed description of the linkage between PICANet and LabBase2 has been reported elsewhere [22]. Briefly, a combination linkage methods were used to identify PICANet admission records that had a corresponding record of BSI in LabBase2, based on agreement between NHS number, hospital number, first name, surname, date of birth, postcode, sex and location (laboratory and hospital) [23].
Case definition
We defined an episode of BSI as any positive blood culture with one or more organisms isolated from any blood sample taken on the same day. Since PICANet does not routinely capture the presence of a CVC during PICU admission, we were unable to determine whether BSI recorded in LabBase2 were related to CVCs or not. Our analysis therefore studied all BSI, and includes any CVC-related or CVC-associated BSI as well as BSI from ventilator-associated pneumonia or catheter-associated urinary tract infection. As a sensitivity analysis, we restricted analyses to children receiving vasoactive agents and requiring invasive ventilation, as these children would be very likely to also require a CVC.
We defined PICU-acquired BSI as any BSI occurring from samples taken from 2 days after admission to 2 days after discharge from PICU. This definition excluded samples taken on the day of admission or the day after admission to PICU, as positive samples on these days may be more reflective of events leading up to admission. We included samples taken on the 2 days following discharge from PICU and so children staying <2 days could have a PICU-acquired BSI if a sample was taken in the 2 days following discharge. This definition may mean that a small proportion of PICU-acquired occurring in the first 2 days of admission are missed (<5 %) [24].
As a secondary analysis, we also evaluated early BSI in PICU (occurring between days 0–7 of admission). This analysis aimed to capture any BSI occurring in the first week of admission (i.e. BSI occurring in the first 2 days of PICU admission and those occurring between days 2–7 of PICU admission). We hypothesised that any effect on BSI of CVC insertion during emergency stabilisation at another hospital could be most apparent within the first week of PICU admission, based on data showing the risk of BSI from insertion of non-cuffed CVCs to be highest in the first few days [25].
Repeated samples with positive cultures of the same organism within 14 days were treated as the same episode. This meant that, for children who already had BSI on admission, we only included PICU-acquired BSI in analysis if the organism from samples taken after admission was a different organism. As laboratory records included the date but not the time the specimen was taken, some included samples may have been taken between 24 and 48 h after admission to PICU.
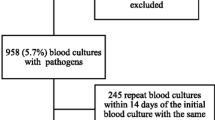
Comparisons of PICU-acquired BSI were restricted to emergency admissions (unplanned admissions as defined within PICANet), since children admitted electively would likely have had a CVC inserted under sterile conditions in the operating theatre. Children with unknown source or type of admission were excluded (Fig. 1).
Statistical analysis
Univariable associations between clinical characteristics and PICU-acquired BSI were assessed using Chi-squared tests (categorical variables) or Mann–Whitney tests (continuous variables). Analysis was performed using Stata 12 [26].
The primary analysis determined whether trends (change over time) in BSI per 1,000 bed-days differed between children with an emergency admission to PICU after inter-hospital transfer compared to within-hospital admission. These analyses used Poisson regression, adjusting for potential confounders including primary diagnosis at admission, ventilation status and Paediatric Index of Mortality (PIM2) score (see Table 1) [27]. Differences in trends in PICU-acquired BSI by source of admission (inter-hospital transfer versus within-hospital admission) were assessed through the inclusion of an interaction term for trend and source of admission.
Time to PICU-acquired BSI was defined as the number of days between admission and the date of the first PICU-acquired BSI. The difference in time to PICU-acquired BSI between inter-hospital and within-hospital transfers was assessed using multivariable Cox regression. Random effects for PICUs were included in models to account for clustering of admissions within PICUs.
We also separately analysed PICU-acquired BSI due to skin and non-skin organisms, as we hypothesised that infection control practices might have had more impact on BSI due to skin organisms acquired during CVC insertion. Skin organisms were defined as coagulase negative staphylococcus, Staphylococcus epidermidis, Corynebacterium spp. or Proprionibacterium spp. All other organisms were classified as non-skin organisms. As BSI could be due to skin organisms, non-skin organisms or both, numbers of BSI due to skin and non-skin organisms exceed the total number of BSI.
Results
Bloodstream infections in PICU
Figure 1 shows the number of records included in the analysis. Admission characteristics are shown in Table 1. For the 62,515 emergency admissions between March 2003 and December 2012, there were 1628 episodes of PICU-acquired BSI, and 1353 admissions (2.2 %) experienced at least one PICU-acquired BSI. This equated to an overall rate of 4.97 (95 % CI 4.73–5.22) PICU-acquired BSI per 1,000 bed-days.
The percentage of admissions acquiring BSI following inter-hospital transfer (56 %) was greater than for within-hospital admissions (42 %). However, after adjusting for significant risk factors (calendar period of admission measured by quarter-year, age, renal support, diagnosis group, and ventilation status, see Table 1 in Supplement) there was no significant difference in rates of PICU-acquired BSI by source of admission. The incidence-rate ratio for inter-hospital transfer versus within-hospital admission was 0.97 (95 % CI 0.87–1.07). There was, however, a significant interaction between source of admission and time, with rates decreasing more rapidly for inter-hospital transfers (17.0 %; 95 % CI 14.9–19.0 % per year) compared with 12.4 % (95 % CI 9.9–14.9 % per year) for within-hospital admissions (p = 0.026).
The sensitivity analysis included 14,393 children who required invasive ventilation and who received vasoactive agents. For these admissions, there were 937 episodes of PICU-acquired BSI, and 763 admissions (5.3 %) experienced at least one PICU-acquired BSI (7.03 per 1,000 bed-days; 95 % CI 6.09–7.97). The incidence-rate ratio for inter-hospital transfer versus within-hospital admission was similar to that in the main analysis: 0.98 (95 % CI 0.86–1.13). In this group, who were likely to have required CVCs, rates also decreased more rapidly for inter-hospital transfers (17.5; 95 % CI 17.0–18.0) compared with 12.6 (95 % CI 10.4–14.5) for within-hospital admissions (p = <0.001).
In the secondary analysis restricting the outcome to early BSI (occurring between days 0–7 of admission), there was no significant difference in rates by source of admission (incidence-rate ratio for inter-hospital transfer vs. within-hospital admission = 0.97; 95 % CI 0.89–1.05). Trends were similar in both groups (p value for interaction term = 0.66).
Overall, non-skin organisms dominated as causative organisms for PICU-acquired BSI (3.76; 95 % CI 3.61–3.91 per 1,000 bed-days for non-skin organisms vs. 1.61; 95 % CI 1.51–1.71 for skin organisms). This was consistent for both inter-hospital transfers and within-hospital admissions. Trends seen in PICU-acquired BSI due to skin organisms and due to non-skin organisms reflected those seen overall (Fig. 2). There was no significant difference in rates or trends of either type of organism between inter-hospital transfers or within-hospital admissions.
Time to PICU-acquired BSI
The percentage of admissions acquiring BSI in PICU increased with length of stay (Table 2). The median time to PICU-acquired BSI did not differ between inter-hospital transfers (7 days from admission; IQR 4–13) and within-hospital admissions (8 days; IQR 4–15). Between 2003 and 2012, there was no significant difference by admission source in time to PICU-acquired BSI (hazard ratio for inter-hospital transfers versus within-hospital admissions = 0.93; 95 % CI 0.83–1.04, Fig. 3) or for early BSI (hazard ratio = 0.96; 95 % CI 0.87–1.06).
The median time to PICU-acquired BSI due to skin organisms was 8 (IQR 4–16) and 7 (IQR 4–13) days for non-skin organisms. There was no significant difference in time to PICU-acquired BSI either due to skin organisms (hazard ratio for inter-hospital transfer vs. within-hospital admissions = 0.90; 95 % CI 0.73–1.13) or non-skin organisms (hazard ratio = 0.91; 95 % CI 0.79–1.04).
Discussion
Our study shows that, in England and Wales, the risk of BSI acquired in PICU did not vary according to whether a child had an emergency admission to PICU following inter-hospital transfer from another hospital or were within-hospital admissions. Previously described risk factors for BSI, such as need for renal support and longer PICU stay, were associated with a greater risk of BSI in our study [10, 12, 28, 29]. Rates of PICU-acquired BSI have continued to fall over the past decade, at a greater rate in inter-hospital transfers than in within-hospital admissions, coincident with several national quality improvement initiatives [18, 19]. Our findings are important in guiding national policy on where future efforts to reduce BSI in PICU should be targeted.
Our study is the first to analyse BSI data at a national level in emergency admissions to PICUs in England and Wales. The main strength of our study was the national coverage of our linked dataset and the long study period of 10 years. We were also able to analyse microbiological data on a large proportion of all PICU admissions during the study period (>70 %). We chose to study all BSI, rather than just BSI related to the presence of a CVC, since children on a PICU are subjected to many invasive procedures, all of which may increase the risk of BSI.
The main weakness of our study, which limits our ability to directly compare our results with previous single-centre studies, was that the linked national dataset did not contain information on which patients had a CVC during their stay in PICU (or where it was inserted and the circumstances surrounding its insertion). As such, any true differences in CVC insertion practice between inter-hospital transfers and within-hospital admissions contributing to the development of PICU BSI could have been masked, biasing our results towards the null. However, since approximately 40–60 % of emergency admissions to PICU require a CVC (unpublished audit data), and since rates of BSI in children without CVCs are low, differences in trends in BSI seen in our study seen between inter-hospital and within-hospital admissions are likely to be related to the use of CVCs. Furthermore, our sensitivity analysis evaluating rates in children requiring vasoactive agents and invasive ventilation showed results almost identical to those in all children, although absolute BSI rates were higher in this group.
There are several potential explanations for our findings. First, the fact that BSI rates decreased more rapidly in the inter-hospital transfer group over the years may be related to differences between the groups studied despite adjustment for case mix and severity of illness, with respect to the type of CVC used (CVCs in inter-hospital transfers were more likely to be short-term non-tunnelled catheters, whereas within-hospital admissions were more likely to have long-term tunnelled catheters), patient susceptibility (within-hospital admissions more likely to have impaired host defence), presence of co-morbidities (more frequent in within-hospital admissions) and length of CVC use. Second, inter-hospital transfers may have benefited more from the implementation of national quality improvement initiatives. Third, the fact that there was no difference in the time to BSI within the first week between the groups may be explained by the use of antibiotics, which may have delayed the onset of insertion-related BSI beyond the first week of admission in inter-hospital transfers.
Our study demonstrates that routine replacement of CVCs inserted in trauma and other emergencies is not justified in critically ill children, as, in England and Wales, children with CVCs inserted following stabilisation are not at a higher risk of BSI. Further, since the rate of BSI has reduced significantly over the past decade, future attempts to eliminate BSI may need to focus more on practice related to the maintenance of CVCs. Studies from other settings have emphasised the importance of CVC maintenance-related care bundles in the reduction of BSI in PICUs, particularly due to the frequent use of tunnelled long-term CVCs in this population [30, 31]. Further research focusing on the influence of co-morbidities, CVC type and exposure, and differential use of antibiotics, on BSI rates in national PICU cohorts is required.
References
Yogaraj JS, Elward AM, Fraser VJ (2002) Rate, risk factors, and outcomes of nosocomial primary bloodstream infection in pediatric intensive care unit patients. Pediatrics 110(3):481–485
Elward AM, Hollenbeak CS, Warren DK, Fraser VJ (2005) Attributable cost of nosocomial primary bloodstream infection in pediatric intensive care unit patients. Pediatrics 115(4):868–872
Nowak JE, Brilli RJ, Lake MR, Sparling KW, Butcher J, Schulte M, Wheeler DS (2010) Reducing catheter-associated bloodstream infections in the pediatric intensive care unit: business case for quality improvement. Pediatr Crit Care Med 11(5):579–587
Nosocomial Infection National Surveillance Service (2003) Surveillance of hospital-acquired bacteraemia in English hospitals, 1997–2002 Public Health Laboratory Service
Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, Sexton B, Hyzy R, Welsh R, Roth G, Bander J, Kepros J, Goeschel C (2006) An intervention to decrease catheter-related bloodstream infections in the ICU. New Engl J Med 355(26):2725–2732. doi:10.1056/NEJMoa061115
Bhutta A, Gilliam C, Honeycutt M, Schexnayder S, Green J, Moss M, Anand K (2007) Reduction of bloodstream infections associated with catheters in paediatric intensive care unit: stepwise approach. BMJ 334(7589):362–365
Costello J, Morrow D, Graham D, Potter-Bynoe G, Sandora T, Laussen P (2008) Systematic intervention to reduce central line-associated bloodstream infection rates in a pediatric cardiac intensive care unit. Pediatrics 121(5):915–923
Jeffries H, Mason W, Brewer M, Oakes K, Muñoz E, Gornick W, Flowers L, Mullen J, Gilliam C, Fustar S (2009) Prevention of central venous catheter-associated bloodstream infections in pediatric intensive care units: a performance improvement collaborative. Infect Control Hosp Epidemiol 30(7):645–651
McKee C, Berkowitz I, Cosgrove SE, Bradley K, Beers C, Perl TM, Winner L, Pronovost PJ, Miller MR (2008) Reduction of catheter-associated bloodstream infections in pediatric patients: experimentation and reality. Pediatr Crit Care Med 9(1):40–46
Costello J, Graham D, Morrow D, Potter-Bynoe G, Sandora T, Laussen P (2009) Risk factors for central line-associated bloodstream infection in a pediatric cardiac intensive care unit. Pediatr Crit Care Med 10(4):453–459
Appelgren P, Hellström I, Weitzberg E, Söderlund V, Bindslev L, Ransjö U (2001) Risk factors for nosocomial intensive care infection: a long-term prospective analysis. Acta Anaesthesiol Scand 45(6):710–719. doi:10.1034/j.1399-6576.2001.045006710.x
Mello M, Albuquerque M, Lacerda H, Souza W, Correia J, Britto M (2009) Risk factors for healthcare-associated infection in pediatric intensive care units: a systematic review. Cad Saúde Pública 25:373–391
Krishnaiah A, Soothill J, Wade A, Mok QQ, Ramnarayan P (2012) Central venous catheter-associated bloodstream infections in a pediatric intensive care unit: effect of the location of catheter insertion. Pediatr Crit Care Med 13(3):e176–e180
Wylie MC, Graham DA, Potter-Bynoe G, Kleinman ME, Randolph AG, Costello JM, Sandora TJ (2010) Risk factors for central line—associated bloodstream infection in pediatric intensive care units. Infect Control Hosp Epidemiol 31(10):1049–1056
Raad II, Hohn DC, Gilbreath BJ, Suleiman N, Hill LA, Bruso PA, Marts K, Mansfield PF, Bodey GP (1994) Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol 15:231–238
O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML (2011) Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis 52(9):e162–e193
Australian Commission on Safety and Quality in Health Care (2012) Central line insertion and maintenance guideline. Australian New Zealand Intensive Care Society, Carlton South
Department of Health (2007) Saving lives: reducing infection, delivering clean and safe care. Department of Health, London
Bion J, Richardson A, Hibbert P, Beer J, Abrusci T, McCutcheon M, Cassidy J, Eddleston J, Gunning K, Bellingan G, Patten M, Harrison D, The Matching Michigan Collaboration Writing Committee (2012) ‘Matching Michigan’: a 2-year stepped interventional programme to minimise central venous catheter-blood stream infections in intensive care units in England. BMJ Qual Saf 22(2):110–123. doi:10.1136/bmjqs-2012-001325
Universities of Leeds and Leicester (2013) Paediatric Intensive Care Audit Network National Report 2011–2013
Wilson J, Elgohari S, Livermore DM, Cookson B, Johnson A, Lamagni T, Chronias A, Sheridan E (2011) Trends among pathogens reported as causing bacteraemia in England, 2004–2008. Clin Microbiol Infect 17(3):451–458. doi:10.1111/j.1469-0691.2010.03262.x
Harron K, Goldstein H, Wade A, Muller-Pebody B, Parslow R, Gilbert R (2013) Linkage, evaluation and analysis of national electronic healthcare data: application to providing enhanced blood-stream infection surveillance in paediatric intensive care. PLoS ONE 8(12):e85278. doi:10.1371/journal.pone.0085278
Goldstein H, Harron K, Wade A (2012) The analysis of record-linked data using multiple imputation with data value priors. Stat Med 31(28):3481–3493
Health Protection Agency (2012) English national point prevalence survey on healthcare-associated infections and antimicrobial use, 2011. www.gov.uk/government/publications/healthcare-associated-infections-hcai-point-prevalence-survey-england (Accessed 06/10/14)
Safdar N, Maki D (2004) The pathogenesis of catheter-related bloodstream infection with noncuffed short-term central venous catheters. Intensive Care Med 30(1):62–67. doi:10.1007/s00134-003-2045-z
Stata (2011) Stata Statistical Software: Release 12. StataCorp, College Station
Visser IE, Hazelzet J, Albers MIJ, Verlaat CM, Hogenbirk K, van Woensel J, van Heerde M, van Waardenburg D, Jansen NG, Steyerberg E (2013) Mortality prediction models for pediatric intensive care: comparison of overall and subgroup specific performance. Intensive Care Med 39(5):942–950. doi:10.1007/s00134-013-2857-4
Harron K, Wade A, Muller-Pebody B, Goldstein H, Parslow R, Gray J, Hartley JC, Mok Q, Gilbert R (2013) Risk-adjusted monitoring of blood-stream infection in paediatric intensive care: a data linkage study. Intensive Care Med 39(6):1080–1087
Tabah A, Koulenti D, Laupland K, Misset B, Valles J, de Carvalho FB, Paiva JA, Çakar N, Ma X, Eggimann P (2012) Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med 38(12):1930–1945
Miller MR, Griswold M, Harris JM II, Yenokyan G, Huskins WC, Moss M, Rice TB, Ridling D, Campbell D, Margolis P, Muething S, Brilli RJ (2010) Decreasing PICU catheter-associated bloodstream infections: nACHRI’s quality transformation efforts. Pediatrics 125(2):206–213. doi:10.1542/peds.2009-1382
Smulders C, van Gestel JJ, Bos A (2013) Are central line bundles and ventilator bundles effective in critically ill neonates and children? Intensive Care Med 39(8):1352–1358. doi:10.1007/s00134-013-2927-7
Acknowledgments
The authors would like to acknowledge Angie Wade and Harvey Goldstein who made substantial contributions to the original data linkage study. The authors would also like to thank Tom Fleming, Phil McShane and Lee Norman (PICANet) for facilitation of data retrieval for this paper. We would like to thank all the staff in participating hospitals who have collected data for PICANet. We are grateful to the UK Paediatric Intensive Care Society for continued support and to the members of the PICANet Steering Group and Clinical Advisory Group who are listed on our website http://www.picanet.org.uk/participants.html. This work was supported by funding for the CATCH trial from the National Institute for Health Research Health Technology Assessment (NIHR HTA) programme (project number 08/13/47). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the HTA programme, NIHR, NHS or the Department of Health. Ruth Gilbert is supported by awards establishing the Farr Institute of Health Informatics Research at UCLP Partners from the MRC and a consortium of funders (MR/K006584/1). PICANet is funded by the National Clinical Audit & Patient Outcomes Programme, administered by the Healthcare Quality Improvement Partnership (HQIP); Welsh Health Specialised Services Committee; NHS Lothian/National Service Division NHS Scotland; the Royal Belfast Hospital for Sick Children; National Office of Clinical Audit Ireland (NOCA) and HCA International.
Ethical approval
For PICANet, collection of personally identifiable data has been approved by the Patient Information Advisory Group (now the NHS Health Research Authority Confidentiality Advisory Group) http://www.hra.nhs.uk/documents/2013/11/piag-register-2.xls and ethical approval granted by the Trent Medical Research Ethics Committee, ref. 05/MRE04/17 +5. PICANet also has specific permission from the National Research Ethics Service for linkage with the PHE laboratory data on bloodstream infections using personal identifiers and to share PICANet data with PHE. An exemption under Sect. 251 of the NHS Act 2006 (previously Sect. 60 of the Health and Social Care Act 2001) allows PHE to receive patient-identifiable data from other organisations without patient consent in order to monitor infectious disease. Specific permission for the PICANet-PHE linkage has been granted by NIGB.
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take-home message:
Evidence underpinning national guidance to replace central venous catheters after admission to intensive care predates major quality improvement initiatives and improved infection control in UK paediatric intensive care units. We used national linked data to demonstrate that inter-hospital transfer is no longer a significant risk factor for bloodstream infection and suggest that strategies to further reduce bloodstream infection should focus on maintaining sterile procedures after admission.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Harron, K., Mok, Q., Parslow, R. et al. Risk of bloodstream infection in children admitted to paediatric intensive care units in England and Wales following emergency inter-hospital transfer. Intensive Care Med 40, 1916–1923 (2014). https://doi.org/10.1007/s00134-014-3516-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3516-0