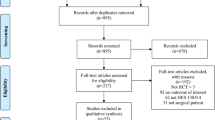
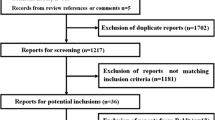
Abstract
Purpose
To determine whether fluid resuscitation of acutely ill adults with 6 % hydroxyethyl starch (6 % HES 130) with a molecular weight of 130 kD and a molar substitution ratio of approximately 0.4 (6 % HES 130) compared with other resuscitation fluids results in a difference in the relative risk of death or treatment with renal replacement therapy (RRT).
Methods
Systematic review and meta-analysis of randomized controlled trials comparing intravascular fluids for resuscitation of hospitalised adults that reported mortality or treatment with RRT. The risk of bias was assessed independently by two reviewers and meta-analysis was performed using random effects.
Results
Thirty-five trials enrolling 10,391 participants were included. The three largest trials had the lowest risk of bias, were published (or completed) in 2012, and together enrolled 77 % of all participants. Death occurred in 928 of 4,691 patients (19.8 %) in the 6 % HES 130 group versus 871 of 4,720 (18.5 %) in the control fluid groups relative risk (RR) in the 6 % HES 130 group 1.08, 95 % confidence interval (CI) 1.00 to 1.17, I 2 = 0 %). Treatment with RRT occurred in 378 of 4,236 patients (8.9 %) in the 6 % HES 130 group versus 306 of 4,260 (7.2 %) in the control fluid group (RR in the 6 % HES 130 group 1.25, 95 % CI 1.08 to 1.44, I 2 = 0 %).
Conclusions
The quality and quantity of data evaluating 6 % hydroxyethyl starch (130/0.4 and 130/0.42) as a resuscitation fluid has increased in the last 12 months. Patients randomly assigned to resuscitation with 6 %HES 130 are at significantly increased risk of being treated with RRT.



Similar content being viewed by others
References
Finfer S, Liu B, Taylor C, Bellomo R, Billot L, Cook D, Du B, McArthur C, Myburgh J (2010) Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care 14:R185
Perel P, Roberts I (2012) Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev 6: CD000567
Reinhart K, Perner A, Sprung CL, Jaeschke R, Schortgen F, Johan Groeneveld AB, Beale R, Hartog CS (2012) Consensus statement of the ESICM task force on colloid volume therapy in critically ill patients. Intensive Care Med 38:368–383
Gattas DJ, Dan A, Myburgh J, Billot L, Lo S, Finfer S (2012) Fluid resuscitation with 6% hydroxyethyl starch (130/0.4) in acutely ill patients: an updated systematic review and meta-analysis. Anesth Analg 114:159–169
Bunn F, Trivedi D (2012) Colloid solutions for fluid resuscitation. Cochrane Database Syst Rev 7: CD001319
Dart AB, Mutter TC, Ruth CA, Taback SP (2010) Hydroxyethyl starch (HES) versus other fluid therapies: effects on kidney function. Cochrane Database Syst Rev: CD007594
Zarychanski R, Turgeon AF, Fergusson DA, Cook DJ, Hébert PC, Bagshaw SM, Monsour D, McIntyre L (2009) Renal outcomes and mortality following hydroxyethyl starch resuscitation of critically ill patients: systematic review and meta-analysis of randomized trials. Open Med 3:E196–E209
Hartog CS, Kohl M, Reinhart K (2011) A systematic review of third-generation hydroxyethyl starch (hes 130/0.4) in resuscitation: safety not adequately addressed. Anesth Analg 112:635–645
Antonelli M, Bonten M, Chastre J, Citerio G, Conti G, Curtis JR, De Backer D, Hedenstierna G, Joannidis M, Macrae D, Mancebo J, Maggiore SM, Mebazaa A, Preiser JC, Rocco P, Timsit JF, Wernerman J, Zhang H (2012) Year in review in Intensive Care Medicine 2011. II. Cardiovascular, infections, pneumonia and sepsis, critical care organization and outcome, education, ultrasonography, metabolism and coagulation. Intensive Care Med 38:345–358
Juni P, Altman DG, Egger M (2001) Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 323:42–46
Higgins JPT GS 16.9.2 Studies with zero-cell counts. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org
Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, Glass P, Lipman J, Liu B, McArthur C, McGuinness S, Rajbhandari D, Taylor CB, Webb SAR (2012) Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. doi:10.1056/NEJMoa1209759
Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Aneman A, Madsen KR, Moller MH, Elkjaer JM, Poulsen LM, Bendtsen A, Winding R, Steensen M, Berezowicz P, Soe-Jensen P, Bestle M, Strand K, Wiis J, White JO, Thornberg KJ, Quist L, Nielsen J, Andersen LH, Holst LB, Thormar K, Kjaeldgaard AL, Fabritius ML, Mondrup F, Pott FC, Moller TP, Winkel P, Wetterslev J (2012) Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med 367:124–134
Siegemund M (2012-09-12) NCT00273728 BaSES trial: Basel Starch Evaluation in Sepsis, personal communication
Gondos T, Marjanek Z, Ulakcsai Z, Szabo Z, Bogar L, Karolyi M, Gartner B, Kiss K, Havas A, Futo J (2010) Short-term effectiveness of different volume replacement therapies in postoperative hypovolaemic patients. Eur J Anaesthesiol 27:794–800
Guidet B, Martinet O, Boulain T, Philippart F, Poussel JF, Maizel J, Forceville X, Feissel M, Hasselmann M, Heininger A, Van Aken H (2012) Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: The CRYSTMAS study. Crit Care 16: R94. Renal replacement results were provided by personal communication, Dr B Guidet, 22 August 2012
Zhu GC, Quan ZY, Shao YS, Zhao JG, Zhang YT (2011) The study of hypertonic saline and hydroxyethyl starch treating severe sepsis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 23:150–153
James MF, Michell WL, Joubert IA, Nicol AJ, Navsaria PH, Gillespie RS (2011) Resuscitation with hydroxyethyl starch improves renal function and lactate clearance in penetrating trauma in a randomized controlled study: the FIRST trial (Fluids in Resuscitation of Severe Trauma). Br J Anaesth 107:693–702
Yang J, Wang WT, Yan LN, Xu MQ, Yang JY (2011) Alternatives to albumin administration in hepatocellular carcinoma patients undergoing hepatectomy: an open, randomized clinical trial of efficacy and safety. Chin Med J (Engl) 124:1458–1464
Nagpal D (2012-08-10) NCT00964015 Starch or Saline After Cardiac Surgery (SSACS) trial, personal communication
Mittermayr M, Streif W, Haas T, Fries D, Velik-Salchner C, Klingler A, Oswald E, Bach C, Schnapka-Koepf M, Innerhofer P, Mittermayr M, Streif W, Haas T, Fries D, Velik-Salchner C, Klingler A, Oswald E, Bach C, Schnapka-Koepf M, Innerhofer P (2007) Hemostatic changes after crystalloid or colloid fluid administration during major orthopedic surgery: the role of fibrinogen administration.[see comment]. Anesth Analg 105:905–917
Schramko A, Suojaranta-Ylinen R, Kuitunen A, Raivio P, Kukkonen S, Niemi T (2010) Hydroxyethylstarch and gelatin solutions impair blood coagulation after cardiac surgery: a prospective randomized trial. Br J Anaesth 104:691–697
Lu J, Zhao HY, Liu F, An YZ (2012) The influence of lactate Ringer solution versus hydroxyethyl starch on coagulation and fibrinolytic system in patients with septic shock. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 24:38–41
Volta CA, Alvisi V, Campi M, Marangoni E, Alvisi R, Castellazzi M, Fainardi E, Manfrinato MC, Dallocchio F, Bellini T (2007) Influence of different strategies of volume replacement on the activity of matrix metalloproteinases: an in vitro and in vivo study. Anesthesiol 106:85–91
Dubin A, Pozo MO, Casabella CA, Murias G, Palizas F, Moseinco MC, Kanoore Edul VS, Estenssoro E, Ince C (2010) Comparison of 6% hydroxyethyl starch 130/0.4 and saline solution for resuscitation of the microcirculation during the early goal-directed therapy of septic patients. J Crit Care 25: 659.e651-659.e658
Van Der Linden PJ, De Hert SG, Deraedt D, Cromheecke S, De Decker K, De Paep R, Rodrigus I, Daper A, Trenchant A (2005) Hydroxyethyl starch 130/0.4 versus modified fluid gelatin for volume expansion in cardiac surgery patients: the effects on perioperative bleeding and transfusion needs. Anesth Analg 101:629–634
Ooi JSM, Ramzisham ARM, Zamrin MD (2009) Is 6% hydroxyethyl starch 130/0.4 safe in coronary artery bypass graft surgery? Asian Cardiovasc Thorac Ann 17:368–372
Zdolsek HJ, Vegfors M, Lindahl TL, Tornquist T, Bortnik P, Hahn RG (2011) Hydroxyethyl starches and dextran during hip replacement surgery: effects on blood volume and coagulation. Acta Anaesthesiol Scand 55:677–685
Wu Y, Wu AS, Wang J, Tian M, Jia XY, Rui Y, Yue Y (2010) Effects of the novel 6% hydroxyethyl starch 130/0.4 on renal function of recipients in living-related kidney transplantation. Chin Med J (Engl) 123:3079–3083
Godet G, Lehot JJ, Janvier G, Steib A, De Castro V, Coriat P (2008) Safety of HES130/0.4 (Voluven(R)) in patients with preoperative renal dysfunction undergoing abdominal aortic surgery: a prospective, randomized, controlled, parallel-group multicentre trial.[erratum appears in Eur J Anaesthesiol. 2008 Dec; 25(12):1042]. Eur J Anaesthesiol 25:986–994
Mahmood A, Gosling P, Vohra RK (2007) Randomized clinical trial comparing the effects on renal function of hydroxyethyl starch or gelatine during aortic aneurysm surgery. Br J Surg 94:427–433
Dolecek M, Svoboda P, Kantorova I, Scheer P, Sas I, Bibrova J, Radvanova J, Radvan M (2009) Therapeutic influence of 20% albumin versus 6% hydroxyethylstarch on extravascular lung water in septic patients: a randomized controlled trial. Hepato-Gastroenterology 56:1622–1628
Schramko AA, Suojaranta-Ylinen RT, Kuitunen AH, Kukkonen SI, Niemi TT (2009) Rapidly degradable hydroxyethyl starch solutions impair blood coagulation after cardiac surgery: a prospective randomized trial. Anesth Analg 108:30–36
Mukhtar A, Aboulfetouh F, Obayah G, Salah M, Emam M, Khater Y, Akram R, Hoballah A, Bahaa M, Elmeteini M, Hamza A, Mukhtar A, Aboulfetouh F, Obayah G, Salah M, Emam M, Khater Y, Akram R, Hoballah A, Bahaa M, Elmeteini M, Hamza A (2009) The safety of modern hydroxyethyl starch in living donor liver transplantation: a comparison with human albumin. Anesth Analg 109:924–930
Inal MT, Memis D, Karamanlioglu B, Sut N, (2010) Effects of polygeline and hydroxyethyl starch solutions on liver functions assessed with LIMON in hypovolemic patients. J crit care 25: 361.e361-365
Palumbo D, Servillo G, D’Amato L, Volpe ML, Capogrosso G, De Robertis E, Piazza O, Tufano R (2006) The effects of hydroxyethyl starch solution in critically ill patients.[see comment]. Minerva Anestesiol 72:655–664
Kasper SM, Meinert P, Kampe S, Gorg C, Geisen C, Mehlhorn U, Diefenbach C (2003) Large-dose hydroxyethyl starch 130/0.4 does not increase blood loss and transfusion requirements in coronary artery bypass surgery compared with hydroxyethyl starch 200/0.5 at recommended doses. Anesthesiol 99:42–47
Gandhi SD, Weiskopf RB, Jungheinrich C, Koorn R, Miller D, Shangraw RE, Prough DS, Baus D, Bepperling F, Warltier DC, Gandhi SD, Weiskopf RB, Jungheinrich C, Koorn R, Miller D, Shangraw RE, Prough DS, Baus D, Bepperling F, Warltier DC (2007) Volume replacement therapy during major orthopedic surgery using Voluven (hydroxyethyl starch 130/0.4) or hetastarch. Anesthesiol 106:1120–1127
Langeron O, Doelberg M, Ang ET, Bonnet F, Capdevila X, Coriat P (2001) Voluven, a lower substituted novel hydroxyethyl starch (HES 130/0.4), causes fewer effects on coagulation in major orthopedic surgery than HES 200/0.5. Anesth Analg 92:855–862
Sander O, Reinhart K, Meier-Hellmann A (2003) Equivalence of hydroxyethyl starch HES130/0. 4 and HES 200/0. 5 for perioperative volume replacement in major gynaecological surgery. Acta Anaesthesiol Scand 47:1151–1158
Gallandat Huet RC, Siemons AW, Baus D, van Rooyen-Butijn WT, Haagenaars JA, van Oeveren W, Bepperling F (2000) A novel hydroxyethyl starch (Voluven) for effective perioperative plasma volume substitution in cardiac surgery. Can J Anaesth 47:1207–1215
Jungheinrich C, Sauermann W, Bepperling F, Vogt NH (2004) Volume efficacy and reduced influence on measures of coagulation using hydroxyethyl starch 130/0.4 (6%) with an optimised in vivo molecular weight in orthopaedic surgery: a randomised, double-blind study. Drugs R D 5:1–9
Mehta Y, Dhar A, Sujatha, Meharwal ZS, Trehan N (2007) Comparison of new HES (130/0.4) and HES (200/0.5) in OPCAB surgery. J Anaesthesiol Clinical Pharmacol 23:273–278
Ellger B, Freyhoff J, Van Aken H, Booke M, Marcus MAE (2006) High-dose volume replacement using HES130/0.4 during major surgery: impact on coagulation and incidence of postoperative itching. Ned Tijdschr Anesthesiol 19:63–68
Neff TA, Doelberg M, Jungheinrich C, Sauerland A, Spahn DR, Stocker R (2003) Repetitive large-dose infusion of the novel hydroxyethyl starch 130/0.4 in patients with severe head injury. Anesth Analg 96:1453–1459
Boldt J, Lehmann A, Rompert R, Haisch G, Isgro F (2000) Volume therapy with a new hydroxyethyl starch solution in cardiac surgical patients before cardiopulmonary bypass. J Cardiothorac Vasc Anesth 14:264–268
Rasmussen LS YS, Schuttler J, Van Aken H, Shafer SL, Eisenach JC, Reilly CS, Miller DR, Zwissler B, Tramer MR, Antonelli M (2011) Editors-in-chief statement regarding irb approval of clinical trials by Joachim Boldt. http://www.aaeditor.org/EICJointStatement.pdf Accessed 20 February 2011
Rasmussen LS YS, Van Aken H, Shafer SL, Eisenach JC, Reilly CS, Miller DR, Parrillo JE, Zwissler B, Tramer MR, Antonelli M, Kaplan JA, Wiltfang J, Stefano GB, Chiumello D, Mayr WR (2011) Editors-in-chief statement regarding published clinical trials conducted without irb approval by Joachim Boldt. http://www.aaeditor.org/EIC.Joint.Statement.on.Retractions.pdf Accessed 7 March 201
Shafer SL (2011) Shadow of doubt. Anesth Analg 112:498–500
Shafer SL, Notice of retraction. http://www.aaeditor.org/NoticeofRetraction.pdf Accessed 20 Feb 2011
Shafer SL (2011) 25 February 2011 notice. Anesthesia & Analgesia. http://www.anesthesia-analgesia.org/site/misc/25.February.2011.Notice.pdf Accessed 1 March 2011
Haynes RB, McKibbon KA, Wilczynski NL, Walter SD, Werre SR (2005) Optimal search strategies for retrieving scientifically strong studies of treatment from medline: analytical survey. BMJ 330:1179
Acknowledgments
The authors acknowledge Dr Nancy Jiang for assistance with language translation. Sources of funding: This study was funded by the Division of Critical Care & Trauma, George Institute for Global Health. JM is supported by a Practitioner Fellowship from the National Health and Medical Research Council.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Crystalloid versus Hydroxyethyl Starch Trial (CHEST) Management Committee: John Myburgh (chair), Rinaldo Bellomo, Alan Cass, Maryam Correa, Simon Finfer, David Gattas, Parisa Glass, Julie Harland, Joanne Lee, Jeffrey Lipman, Bette Liu, Colin McArthur, Shay McGuinness, Maryanne Ng, Dorrilyn Rajbhandari, Colman Taylor, Steve Webb and Lynsey Willenberg.
This article is discussed in the editorial available at doi:10.1007/s00134-013-2854-7.
Appendix
Appendix
Electronic Search strategy. The intersection of: fluid resuscitation, hydroxyethyl starch, and randomized controlled trials.
MEDLINE.
1. exp Fluid Therapy.
2. ((fluid$ or volume$ or plasma$ or rehydrat$) adj3 (replace$ or therap$ or substitut$ or restor$ or resuscitat$ or rehydrat$)).ab,ti.
3. or/1-2.
4. exp Starch.
5. exp Blood Substitutes.
6. exp Colloids.
7. hetastarch$.tw.
8. hydroxyethyl starch.tw.
9. hydroxyethylstarch.tw.
10. hydroxy ethyl starch.tw.
11. pentastarch.tw.
12. voluven$.tw.
13. tetrastarch.tw.
14. or/4-13.
15. 3 and 14.
16. limit 15 to “therapy (sensitivity)” [from the MEDLINE limit ‘Clinical Queries’, based on Haynes et al [52].].
EMBASE.
#14. #3 AND #12 AND #13.
#13. random:ti OR ‘clinical trial’:de,rn,ab,ti OR ‘health care quality’/exp.
#12. #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11.
#11. tetrastarch.
#10. voluven*.
#9. pentastarch.
#8. ‘hydroxy ethyl starch’.
#7. hydroxyethylstarch.
#6. ‘hydroxyethyl starch’.
#5. hetastarch*.
#4. ‘starch’/exp OR starch.
#3. #1 OR #2.
#2. (fluid* OR volum* OR plasma* OR rehydrat*) NEAR/3 (therap* OR substitut* OR restor* OR resusc* OR replac*).
#1. ‘fluid therapy’/exp OR ‘fluid therapy’.
CENTRAL.
#1 starch* or *starch or voluven* in Clinical Trials.
#2 MeSH descriptor Fluid Therapy explode all trees.
#3 ((fluid* or volume* or plasma* or rehydrat*) NEAR/3 (replace* or therap* or substitut* or restor* or resuscitat* or rehydrat*)):ab,ti in Clinical Trials.
#4 (#2 OR #3).
#5 (#1 AND #4).
Rights and permissions
About this article
Cite this article
Gattas, D.J., Dan, A., Myburgh, J. et al. Fluid resuscitation with 6 % hydroxyethyl starch (130/0.4 and 130/0.42) in acutely ill patients: systematic review of effects on mortality and treatment with renal replacement therapy. Intensive Care Med 39, 558–568 (2013). https://doi.org/10.1007/s00134-013-2840-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-013-2840-0