Abstract
Purpose
Our aim was to identify the clinical profile of intensive care unit (ICU) patients with Pseudomonas aeruginosa (PA) pneumonia and the impact on ICU mortality and duration of mechanical ventilation (MV) of multidrug resistance (MDR) in the PA isolate and inadequate initial antibiotic therapy (IIAT).
Methods
We conducted a retrospective analysis of data prospectively collected in the 18-bed general ICU of a major teaching hospital in Rome, Italy. The study cohort consisted of 110 adult patients with culture-confirmed PA pneumonia consecutively diagnosed in 2008–2010. ICU survivor and nonsurvivor groups were compared to identify factors associated with ICU mortality.
Results
In 42 (38 %) of the 110 cases of PA pneumonia analyzed, the PA isolate was MDR. Fifty-six (50.9 %) of the patients received IIAT, and 49 (44.5 %) died in ICU. In logistic regression analysis, IIAT, diabetes mellitus, higher Simplified Acute Physiology Score (SAPS) II scores, and older age were independently associated with ICU mortality. Among survivors, those who received IIAT or had MDR PA pneumonia had significantly longer median (interquartile ranges, IQR) periods of post-pneumonia onset MV (16.5 [14.5–20] and 15 [12–18] days, respectively) compared with those whose initial therapy was adequate (8 [6–13] days, P < 0.001) and those whose infections were caused by non-MDR PA (10.5 [6.5–13] days, P = 0.01).
Conclusions
Our findings highlight the importance of IIAT as a risk factor for mortality in ICU patients with PA pneumonia. MDR in the PA isolate, like IIAT, can significantly increase the need for MV.
Similar content being viewed by others
Introduction
Pseudomonas aeruginosa (PA) is one of the Gram-negative pathogens most commonly isolated in patients with health-care-associated pneumonia (HCAP), hospital-acquired pneumonia (HAP) (in intensive care unit [ICU] and non-ICU patients), or ventilator-associated pneumonia (VAP), and its incidence as a nosocomial lung pathogen has doubled over the last three decades [1–4]. PA is intrinsically resistant to several antimicrobial agents, and it can acquire resistance to many others. The frequency of multidrug-resistant (MDR) strains is increasing, especially in nosocomial and ICU-acquired infections [5–7], and these infections can be associated with increases in mortality, morbidity, and hospital costs. Delays in initiating appropriate antibiotic therapy could obviously increase the rates of treatment failure and attributable mortality [8–10], but even if effective treatment is started promptly, up to 43 % of patients with PA VAP die [11, 12].
The risk factors for PA pneumonia and the major determinants of its outcome in ICU patients have been investigated by various groups [13–21]. However, most have focused mainly on VAP and/or infections acquired in the ICU, and none have explored the impact on outcomes of multidrug-resistance in the PA strain causing the infections. To address these gaps, we analyzed the clinical profile of a cohort of critically ill ICU patients with PA pneumonia. The impact of adequacy of initial antibiotic treatment on mortality and duration of mechanical ventilation were also evaluated.
Methods
Setting and study design
This retrospective cohort study was conducted in the 1500-bed Medical Center of the Catholic University of the Sacred Heart in Rome, Italy; the protocol was approved by the University’s Ethics Committee, and informed consent was waived because of the observational retrospective nature of this study. We searched the ICU’s clinical and microbiological databases to identify all cases of PA pneumonia diagnosed between January 1, 2008, through December 31st, 2010, that met the following criteria: patient age ≥18 years; pneumonia onset shortly before (≤24 h) or after admission to the medical center’s general ICU; diagnoses confirmed by quantitative culture of bronchoalveolar lavage fluid (BAL) showing PA growth of >104 cfu/ml.
HAP was diagnosed when a new, persistent, progressive radiographic lung infiltrate, that was not evident within the first 48 h following hospital admission, and two or more of the following clinical criteria were met: (1) new onset of purulent bronchial sputum, (2) body temperature >38.8 °C or <35.5 °C, (3) white blood cell count >10,000/mm3 or <4,000/mm3. VAP was defined as ICU-acquired pneumonia occurring ≥ 48 h after endotracheal intubation and/or mechanical ventilation. HCAP was defined as pneumonia developed in patients who (1) were hospitalized in an acute care hospital for two or more days within 90 days of the infection; (2) were residents in a nursing home or long-term care facility; (3) had received intravenous antibiotic therapy, chemotherapy, or wound care within the 30 days preceding the index infection; or (4) were in regular hemodialysis treatment.
Cases of VAP were excluded if the patient’s retrospectively calculated Clinical Pulmonary Infection Score (CPIS) was <6 [22].
Survivor and nonsurvivor subgroups were compared to identify factors associated with ICU mortality. Patient data (collected from electronic medical records and laboratory databases) included demographic characteristics, medical history, clinical and laboratory findings, the Simplified Acute Physiology Score II (SAPS II) and Sequential Organ Failure Assessment (SOFA) score calculated by ICU physicians at the time of ICU admission and at onset of pneumonia, respectively; treatment; and outcome [23, 24].
Microbiological analyses
BAL specimens were collected blindly with telescopic catheters (Combicath; Plastimed, Saint-Leu-la-Forêt, France) in almost all patients (with the exception of eight patients for whom a bronchoscopic guide was required) and a calibrated loop of aspirate (0.0025 mL) was quantitatively cultured on blood agar, chocolate agar, McConkey agar, and when necessary Legionella agar (blood charcoal yeast extractB). Three or more sets of blood cultures had also been collected for each patients in Lytic/10 Anaerobic/F and Plus Aerobic/F bottles and incubated in the automated Bactec 9240 system (all from Becton–Dickinson, Sparks, MD). Bacterial isolates were identified with the VITEK 2 (bioMérieux, Inc., Hazelwood, MO) and/or Phoenix (Becton–Dickinson) systems. All isolates were subjected to E-testing (bioMérieux) and minimum inhibitory concentrations (MICs) were classified according to current (2008) Clinical Laboratory Standards Institute (CLSI) guidelines [25].
Definitions
PA isolates were classified as MDR if they displayed in vitro resistance to at least 1 antipseudomonal agent in 3 or more of the following categories: β-lactam/β-lactamase inhibitors (i.e., piperacillin–tazobactam); cephalosporins (i.e., cefepime and ceftazidime); carbapenems (i.e., imipenem and meropenem); quinolones (i.e., ciprofloxacin and levofloxacin); and aminoglycosides (i.e., amikacin and gentamicin) [8]. Colistin-only-susceptible (COS) PA strains were resistant to all commercially available drugs except colistin.
Pneumonia onset coincided with the collection date of the first BAL culture yielding the study isolate (index culture), and septic shock was defined as recommended by the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee [26]. We recorded the length (days) of the period at risk for PA pneumonia (i.e., from hospital admission to pneumonia onset); the total ICU stay and the post-pneumonia-onset ICU stay (both ending at ICU discharge or death, whichever occurred first); and the duration of mechanical ventilation [MV] before pneumonia onset (when applicable) and after pneumonia onset. Prior antimicrobial therapy was defined as the use of any antimicrobial for ≥48 h during the 3 months preceding pneumonia onset. The empirical antimicrobial regimen (i.e., that used before in vitro susceptibility data were available for the BAL isolate) was classified as inadequate when it did not include any agent displaying in vitro activity against the isolated pathogen.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) or medians and interquartile ranges [IQR]. The Student t test and Mann–Whitney U test were used to assess normally and non-normally distributed continuous variables, respectively. Categorical variables were reported as percentages of the group analyzed and assessed with the χ2 or two-tailed Fisher exact test. Odds ratios (ORs) and 95 % confidence intervals (CIs) were calculated for all associations.
Logistic regression was used to identify independent predictors of ICU mortality. Variables associated with mortality in the univariate analysis (P ≤0.2) were included at model entry, and a backward stepwise approach was used to identify independent predictors of mortality. Variables with p values ≤0.05 were retained in the final model. The Kaplan–Meier method was used for the survival analysis. Two-tailed tests were used to determine significance; P < 0.05 was considered significant. Analyses were performed with the Intercooled Stata program, version 11.
Results
Incidence and population characteristics
Of the 2,136 patients cared for in our ICU during the 3-year study period, 110 had diagnoses of PA pneumonia (overall incidence: 51.5 per 1,000 ICU admissions). In Fig. 1 the flow chart of study inclusion process is reported.
Twenty nine (26.4 %) patients were admitted to the ICU for recent-onset pneumonia acquired elsewhere in the hospital (non-ICU-acquired pneumonia), and all 29 were placed on MV at ICU admission. In the other 81 (73.6 %) cases, pneumonia onset occurred in the ICU. Seventy-one of these patients were already being ventilated at pneumonia onset, and the other 10 were placed on MV at pneumonia onset. The characteristics of these two subgroups are summarized in Table 1.
Resistance profiles of PA isolates
Forty-eight (43.6 %) of the 110 PA isolates displayed in vitro resistance to ceftazidime, 46 (41.8 %) were resistant to cefepime, 47 (42.7 %) to levofloxacin and ciprofloxacin, 47 (42.7 %) to imipenem, 45 (40.9 %) to meropenem, 33 (30 %) to gentamicin, 23 (20.9 %) to piperacillin–tazobactam, 12 (10.9 %) to amikacin, and none were resistant to colistin. Forty-two strains (38 %) were classified as MDR-PA and 9 (8.1 %) as COS-PA.
Treatment
Promptly (i.e., within few hours) after pneumonia onset, all patients were being empirically treated with anti-Gram-negative drugs (alone or with other antibiotics). In particular, the doses used for the most utilized antimicrobials were: piperacillin/tazobactam 18 g q 24 h (continuous infusion) or 4.5 g every 6 h; meropenem 2 g every 8 h (infusion duration at least 3 h); ceftazidime or cefepime 2 g every 8 h; gentamicin 5–7 mg/kg q 24 h, amikacin 15–20 mg q 24 h, ciprofloxacin 400 every 8 h; and colistin 6 000 000–9 000 000 IU/day divided into 2–3 daily doses. All dosages were adjusted for creatinine clearance if necessary.
The antibiotic regimens, empirical or definitive (i.e., post-antibiogram) are shown in Table 3. The overall mean duration of treatment was 12 ± 4 days. For 65 patients (59 %) antibiotic therapy consisted of a single drug and in 45 (40.9 %) of a combination therapy in the empirical phase. The more common second antibiotics used were amikacin (31.1 %), ciprofloxacin (28.9 %), and colistin (24.4 %). The combination therapy was used in 30 % of patients in the definitive phase of treatment. In 11 patients, the definitive drug regimens (based on in vitro susceptibility data) included aerosol therapy with the same drug used intravenously (i.v.) (i.e., colistin or amikacin); 7 of these 11 patients received i.v. monotherapy with colistin because the infection was caused by strains susceptible only to colistin.
The initial antimicrobial regimen was inadequate in 56 (50.9 %) of the 110 cases, and most IIAT regimens (41/56–73.2 %) included only one drug. IIAT was significantly more common among patients with MDR PA pneumonia (66.6 % [28/42] versus 41.2 % [28/68] in the non-MDR subgroup; P = 0.009). Antibiograms were reported approximately 62 h (median) after pneumonia onset (IQR 48–76 h), and shortly thereafter 46 of the 56 patients were switched to adequate regimens. The other 10 died before their treatment could be adjusted.
Factors associated with inadequate initial antimicrobial treatment
Fifty-six patients who received IIAT were compared with 54 who received adequate antimicrobial therapy from pneumonia onset in order to identify risk factors for IIAT (Table 2). Patients in IIAT group were more likely to be admitted in for ICU medical conditions, to have received a carbapenem-based empirical treatment, and to have a MDR PA pneumonia; Conversely, patients adequately treated, more frequently were admitted to the ICU for trauma and received a β-lactam/β-lactamase inhibitors-based regimen and/or a combination therapy in the empirical phase of treatment.
In logistic regression analysis infection caused by MDR PA isolates resulted independently associated with IIAT (OR, 2.67; 95 % CI, 1.16–6.16; P = 0.02), whereas the use of combination therapy in the empirical phase was associated with a lower risk of IIAT (OR, 0.31; 95 % CI, 0.14–0.70; P = 0.005).
Outcomes
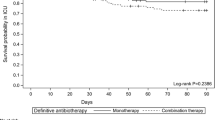
The overall ICU mortality was 44.5 % (49/110) and in-hospital mortality was 47.3 % (52/110). Mortality in the IIAT subgroup was significantly higher than that of the subgroup treated appropriately from the onset: 64.2 % (36/56) versus 24.7 % (13/54) (P < 0.001). Survival curve analysis confirmed the increased mortality risk associated with IIAT (P < 0.001) (Fig. 2). ICU mortality was also significantly higher in patients with MDR PA infections: 59.5 % (25/42) versus 35.2 % (24/68) in the non-MDR group, (P = 0.01), but survival curves in these two subgroups were not significantly different.
We assessed the impact of both these factors on the length of post-pneumonia-onset MV. In the subgroup that survived, significantly longer periods of ventilation were associated with IIAT (16.5 [14.5–20] days versus 8 [6–13] days in those with adequate empirical therapy; P < 0.001) and with MDR PA pneumonia (15 [12–18] days versus 10.5 [6.5–13] days in patients with non-MDR isolates; P = 0.01) (Fig. 3). Less evident differences were observed when the entire cohort was included in this analysis.
Days of mechanical ventilation after P. aeruginosa pneumonia onset in ICU survivors: significantly longer ventilation periods were associated with inadequate initial antibiotic treatment (P = <0.001) and PA isolates with multi-drug resistant (MDR) phenotypes (P = 0.01). Boxes represent interquartile ranges (lower border 25th percentile; upper border 75th percentile) and median (50th percentile) (horizontal line within the box; whiskers indicate minimum and maximum values
Risk factors for mortality
Univariate analysis revealed significant differences between the survivor and nonsurvivor subgroups (Table 3). In general, nonsurvivors were older with more comorbidities and more severe illness at pneumonia onset. Their pneumonia was also more likely to be caused by an MDR PA strain. IIAT and carbapenem-based empirical regimens were observed more frequently in the nonsurvivor group. Survival was associated with admissions for trauma, tracheotomy, and empirical regimens that were based on β-lactams/β-lactamase inhibitors and/or included two or more drugs. In logistic regression analysis, only four of these factors were independently associated with ICU mortality: IIAT (OR, 7.89; 95 % CI, 2.61–23.85; P < 0.001), diabetes mellitus (OR, 5.46; 95 % CI, 1.05–28.42; P = 0.04), higher SAPS II (OR, 1.05; 95 % CI, 1.01-1.09; P = 0.01), and older age (OR, 1.05; 95 % CI, 1.01–1.08; P = 0.01).
Discussion
PA pneumonia in an ICU patient is still a life-threatening infection. The ICU mortality rate in our cohort was 44.5 %, which is in line with previous observations [7, 15, 17, 27].
About 80 % of the PA isolates in our study were significantly resistant to one or more of the antimicrobials tested, and 38 % were classified as MDR. In univariate analysis, MDR was strongly correlated with ICU mortality, but this association was not confirmed by multivariate analysis. This finding contrasts with previously reported findings regarding MDR PA BSIs [8, 28, 29], and also with observations on other types of antibiotic-resistant bacteria [30], but the discrepancy might stem from differences in the case mixes (unlike the present study, the ones investigating BSIs also included patients who were not being cared for in the ICU).
IIAT was the most important independent risk factor for mortality and also the only one that is modifiable, and this association was confirmed by survival curve analysis. A recent meta-analysis found that IIAT more than doubles the odds of mortality in patients with VAP [31]. A more recent review examined 14 studies investigating the impact of IIAT in ICU patients with VAP (related to PA in 14.4–100 % of the cases analyzed). IIAT was significantly linked to mortality in only eight of the 14 studies. The authors suggested that the negative findings in the other six might reflect insufficient statistical power or the presence of very severe infections that were fatal despite timely and effective antimicrobial treatment [7].
Not surprisingly, in our study IIAT was independently related to the antimicrobial susceptibility profile of the PA isolate, and MDR PA isolation more than doubled the likelihood of IIAT (OR 2.67). The antimicrobial susceptibility pattern of the infecting organism is obviously not available when empirical therapy is being prescribed. In its stead, however, an epidemiological profile of the ICU itself can do much to improve the choice of an empirical regimen, even if the impact of this information on prescribing practices depends on the way it is used.
The use of antibiotics’ combination in the empirical phase of treatment was the only factor significantly associated with a low risk of IIAT, and this is in line with the observation of Garnacho-Montero et al. [10] who found that in patients with PA VAP the odds of IIAT were reduced by use of combined-drug rather than single-drug empirical regimens. In contrast, in this latter study, monotherapy and combined-drug regimens with effective drugs produced similar outcomes during the non-empirical phase, and again this observation is similar to our results.
Given the high mortality rate of PA pneumonia and the shrinking pipeline for new drugs to combat it, recent efforts have been made to make better use of the drugs currently at our disposal. Several groups have highlighted the effects of ureido-/carboxypenicillin (e.g., piperacillin-tazobactam, PTZ) resistance on IIAT rates for PA pneumonia in ICU patients [15–17], but the actual impact of any antipseudomonal agent will obviously depend on local resistance rates. For example, the EPIC I study found that 37 % of the PA isolates recovered in European ICUs were resistant to ureidopenicillins [32], which suggests that PTZ monotherapy is a poor option for empirical treatment of severe infections that may be caused by PA. In our unit, however, while MDR PA is also fairly common, the PTZ resistance rate—about 20 %—is roughly half that observed for the antipseudomonal cephalosporis, carbapenems, and fluoroquinolones, so in units like ours, empirical regimens based on antipseudomonal drug combinations that include PTZ should reduce IIAT rates. In addition, in our cohort, antibiogram-driven regimens that were PTZ-based (used in continuous infusion in about one-third of patients) were associated with mortality rates identical to those observed with carbapenem-based therapy (14/36, 38.9 % vs. 12/30, 38.7 %). PTZ might thus be a suitable option for definitive therapy of PA pneumonia in certain ICUs, and this solution could stem the rise in carbapenem consumption, which is particularly worrisome in ICU settings where carbapenemase-producing organisms are becoming more and more common [33]. Definitive PTZ-based therapy is even more likely to be successful in the future since the susceptibility breakpoint for this drug has recently been lowered [34].
In our cohort, patients with MDR PA pneumonia and those who had received IIAT also had significantly prolonged post-infection mechanical ventilation times and consequently, longer post-infection ICU stays, an important determinant of total ICU costs [9]. The impact of antibiotic resistance on the length of hospital stays and costs has been widely demonstrated for severe infections caused by Gram-negative bacteria, including PA [35–37], and for VAP caused by methicillin-resistant Staphylococcus aureus (MRSA) [38]. Similarly, IIAT has been linked to longer hospital stays and higher costs in cases of severe sepsis and septic shock related to gram-negative organisms and MRSA sterile-site infections [39, 40]. However, limited data are available on the specific impact of antibiotic resistance or IIAT on ICU stays in patients diagnosed with PA pneumonia. Trouillet et al. found no difference between VAPs caused by piperacillin-resistant and piperacillin-susceptible PA in terms of the post-pneumonia duration of MV [17]. Kollef et al. [9] studied patients with VAP attributed to potentially antibiotic-resistant Gram-negative bacteria and found that post-infection MV and ICU stays were unrelated to the appropriateness of the initial antibiotic regimen. Aside from differences in population characteristics, the discordance between their results and those of our study might be partly related to the fact that we assessed the length of MV and ICU care only in patients who survived (since both of these periods would be shortened rather than lengthened by early death).
Our study has some limitations that have to be acknowledged. Firstly, it was conducted in a single center. Secondly, the retrospective nature of the study could underestimate the role of certain factors. Thirdly, despite the large number of variables included in our analysis, other factors not studied might have influenced the results.
In conclusion, our findings confirm that PA pneumonia is associated with high mortality in ICU patients and highlight the importance of IIAT as a risk factor for this outcome. MDR in the PA isolate is not independently related to ICU mortality in these cases, but, like IIAT, it can significantly increase the length of MV. Our findings also suggest that, depending on local resistance patterns, PTZ-based regimens may be suitable in the empirical and definitive phases of treatment in these cases, an option that could reduce carbapenem consumption in ICU settings.
References
Goossens H (2003) Susceptibility of multi-drug-resistant Pseudomonas aeruginosa in intensive care units: results from the European MYSTIC study group. Clin Microbiol Infect 9:980–983
Parker CM, Kutsogiannis J, Muscedere J, Cook D, Dodek P, Day AG, Heyland DK, Canadian Critical Care Trials Group (2008) Ventilator-associated pneumonia caused by multidrug-resistant organisms or Pseudomonas aeruginosa: prevalence, incidence, risk factors, and outcomes. J Crit Care 23:18–26
Jones RN (2010) Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis 51(Suppl 1):S81–S87
Torres A, Ewig S, Lode H, Carlet J (2009) Defining, treating and preventing hospital acquired pneumonia: European perspective. Intensive Care Med 35:9–29
Mesaros N, Nordmann P, Plésiat P, Roussel-Delvallez M, Van Eldere J, Glupczynski Y, Van Laethem Y, Jacobs F, Lebecque P, Malfroot A, Tulkens PM, Van Bambeke F (2007) Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin Microbiol Infect 13:560–578
Riou M, Carbonnelle S, Avrain L, Mesaros N, Pirnay JP, Bilocq F, De Vos D, Simon A, Piérard D, Jacobs F, Dediste A, Tulkens PM, Van Bambeke F, Glupczynski Y (2010) In vivo development of antimicrobial resistance in Pseudomonas aeruginosa strains isolated from the lower respiratory tract of Intensive Care Unit patients with nosocomial pneumonia and receiving antipseudomonal therapy. Int J Antimicrob Agents 36:513–522
Sun HY, Fujitani S, Quintiliani R, Yu VL (2011) Pneumonia due to Pseudomonas aeruginosa: part II: antimicrobial resistance, pharmacodynamic concepts, and antibiotic therapy. Chest 139:1172–1185
Tumbarello M, Repetto E, Trecarichi EM, Bernardini C, DE Pascale G, Parisini A, Rossi M, Molinari MP, Spanu T, Viscoli C, Cauda R, Bassetti M (2011) Multidrug-resistant Pseudomonas aeruginosa bloodstream infections: risk factors and mortality. Epidemiol Infect 139:1740–1749
Kollef KE, Schramm GE, Wills AR, Reichley RM, Micek ST, Kollef MH (2008) Predictors of 30-day mortality and hospital costs in patients with ventilator-associated pneumonia attributed to potentially antibiotic-resistant gram-negative bacteria. Chest 134:281–287
Garnacho-Montero J, Sa-Borges M, Sole-Violan J, Barcenilla F, Escoresca-Ortega A, Ochoa M, Cayuela A, Rello J (2007) Optimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: an observational, multicenter study comparing monotherapy with combination antibiotic therapy. Crit Care Med 35:1888–1895
Fagon JY, Chastre J, Hance AJ, Montravers P, Novara A, Gibert C (1993) Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med 94:281–288
Montravers P, Fagon JY, Chastre J, Lecso M, Dombret MC, Trouillet JL, Gibert C (1993) Follow-up protected specimen brushes to assess treatment in nosocomial pneumonia. Am Rev Respir Dis 147:38–44
Azoulay E, Timsit JF, Tafflet M, de Lassence A, Darmon M, Zahar JR, Adrie C, Garrouste-Orgeas M, Cohen Y, Mourvillier B, Schlemmer B, Outcomerea Study Group (2006) Candida colonization of the respiratory tract and subsequent pseudomonas ventilator-associated pneumonia. Chest 129:110–117
Yang K, Zhuo H, Guglielmo BJ, Wiener-Kronish J (2009) Multidrug-resistant Pseudomonas aeruginosa ventilator-associated pneumonia: the role of endotracheal aspirate surveillance cultures. Ann Pharmacother 43:28–35
Kaminski C, Timsit JF, Dubois Y, Zahar JR, Garrouste-Orgeas M, Vesin A, Azoulay E, Feger C, Dumenil AS, Adrie C, Cohen Y, Allaouchiche B, OUTCOMEREA study group (2011) Impact of ureido/carboxypenicillin resistance on the prognosis of ventilator-associated pneumonia due to Pseudomonas aeruginosa. Crit Care 15:R112
Combes A, Luyt CE, Fagon JY, Wolff M, Trouillet JL, Chastre J (2006) Impact of piperacillin resistance on the outcome of Pseudomonas ventilator-associated pneumonia. Intensive Care Med 32:1970–1978
Trouillet JL, Vuagnat A, Combes A, Kassis N, Chastre J, Gibert C (2002) Pseudomonas aeruginosa ventilator-associated pneumonia: comparison of episodes due to piperacillin-resistant versus piperacillin-susceptible organisms. Clin Infect Dis 34:1047–1054
El Solh AA, Akinnusi ME, Wiener-Kronish JP, Lynch SV, Pineda LA, Szarpa K (2008) Persistent infection with Pseudomonas aeruginosa in ventilator-associated pneumonia. Am J Respir Crit Care Med 178:513–519
Giantsou E, Manolas K (2011) Superinfections in Pseudomonas aeruginosa ventilator-associated pneumonia. Minerva Anestesiol 77:964–970
Venier AG, Gruson D, Lavigne T, Jarno P, L’hériteau F, Coignard B, Savey A, Rogues AM, REA-RAISIN group (2011) Identifying new risk factors for Pseudomonas aeruginosa pneumonia in intensive care units: experience of the French national surveillance, REA-RAISIN. J Hosp Infect 79:44–48
Rello J, Allegri C, Rodriguez A, Vidaur L, Sirgo G, Gomez F, Agbaht K, Pobo A, Diaz E (2006) Risk factors for ventilator-associated pneumonia by Pseudomonas aeruginosa in presence of recent antibiotic exposure. Anesthesiology 105:709–714
Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM (1991) Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis 143:1121–1129
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL (2001) Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 286:1754–1758
Clinical Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; Eighteenth Informational Supplement. 2008, M100-S18. Wayne, PA
Dellinger RP, Levy MM, Carlet JM et al (2008) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36:296–327
Fujitani S, Sun HY, Yu VL, Weingarten JA (2011) Pneumonia due to Pseudomonas aeruginosa: part I: epidemiology, clinical diagnosis, and source. Chest 139:909–919
Tam VH, Rogers CA, Chang KT, Weston JS, Caeiro JP, Garey KW (2010) Impact of multidrug-resistant Pseudomonas aeruginosa bacteremia on patient outcomes. Antimicrob Agents Chemother 54:3717–3722
Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, Oh MD, Choe KW (2005) Risk factors for antimicrobial resistance and influence of resistance on mortality in patients with bloodstream infection caused by Pseudomonas aeruginosa. Microb Drug Resist 11:68–74
Damas P, Layios N, Seidel L, Nys M, Melin P, Ledoux D (2011) Severity of ICU-acquired pneumonia according to infectious microorganisms. Intensive Care Med 37:1128–1135
Kuti EL, Patel AA, Coleman CI (2008) Impact of inappropriate antibiotic therapy on mortality in patients with ventilator-associated pneumonia and blood stream infection: a meta-analysis. J Crit Care 23:91–100
Vincent JL (2000) Microbial resistance: lessons from the EPIC study. European Prevalence of Infection. Intensive Care Med 26(Suppl 1):S3–S8
Nordmann P, Naas T, Poirel L (2011) Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 17:1791–1798
Clinical Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 22th Informational Supplement. 2012, M100-S22. Wayne, PA
Giske CG, Monnet DL, Cars O, Carmeli Y, ReAct-Action on Antibiotic Resistance (2008) Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob Agents Chemother 52:813–821
Tumbarello M, Spanu T, Di Bidino R, Marchetti M, Ruggeri M, Trecarichi EM, De Pascale G, Proli EM, Cauda R, Cicchetti A, Fadda G (2010) Costs of bloodstream infections caused by Escherichia coli and influence of extended-spectrum-beta-lactamase production and inadequate initial antibiotic therapy. Antimicrob Agents Chemother 54:4085–4091
Mauldin PD, Salgado CD, Hansen IS, Durup DT, Bosso JA (2010) Attributable hospital cost and length of stay associated with health care-associated infections caused by antibiotic-resistant gram-negative bacteria. Antimicrob Agents Chemother 54:109–115
Shorr AF, Combes A, Kollef MH, Chastre J (2006) Methicillin-resistant Staphylococcus aureus prolongs intensive care unit stay in ventilator-associated pneumonia, despite initially appropriate antibiotic therapy. Crit Care Med 34:700–706
Shorr AF, Micek ST, Kollef MH (2008) Inappropriate therapy for methicillin-resistant Staphylococcus aureus: resource utilization and cost implications. Crit Care Med 36:2335–2340
Shorr AF, Micek ST, Welch EC, Doherty JA, Reichley RM, Kollef MH (2011) Inappropriate antibiotic therapy in Gram-negative sepsis increases hospital length of stay. Crit Care Med 39:46–51
Acknowledgments
This study was partially supported by a grant from the Università Cattolica del Sacro Cuore, Linea D1 2011.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tumbarello, M., De Pascale, G., Trecarichi, E.M. et al. Clinical outcomes of Pseudomonas aeruginosa pneumonia in intensive care unit patients. Intensive Care Med 39, 682–692 (2013). https://doi.org/10.1007/s00134-013-2828-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-013-2828-9