Abstract
Purpose
The aim of this study is to assess the incidence of sublingual microcirculatory flow alterations, according to a predefined arbitrary cutoff value, in patients with “clinical signs of impaired organ perfusion”. Secondary endpoints were the changes in microvascular flow index (MFI), “clinical signs of impaired organ perfusion”, and stroke volume (SV) after fluid administration, and the differences between groups.
Methods
Prospective, single-center, observational study in a 22-bed mixed intensive care unit (ICU). Patients ≥18 years with invasive hemodynamic monitoring and “clinical signs of impaired organ perfusion” as the principal reason for fluid administration were included. Before and after fluid challenge, systemic hemodynamics and direct in vivo observation of the sublingual microcirculation with sidestream dark-field imaging were obtained. Microvascular flow index (MFI) <2.6 was predefined as abnormal.
Results
N = 50. At baseline, MFI <2.6 was present in 66 % of the patients. In these patients, MFI increased from 2.3 (2–2.5) at baseline to 2.5 (2.1–2.8) after fluid challenge (p = 0.003). This was accompanied by a reduction in the number of “clinical signs of impaired organ perfusion” from 2 (1–2) to 1 (0–2) (p < 0.001). However, in patients with MFI >2.6 at baseline, MFI and clinical signs changed insignificantly [2.8 (2.8–2.9) versus 2.8 (2.7–3), p = 0.45, respectively, 1 (1–2) versus 1 (1–2), p < 0.32]. These changes were not restricted to patients with a rise in SV ≥10 %.
Conclusions
These data add to the understanding that noninvasive assessment of microvascular blood flow may help to identify patients eligible for fluid therapy, and to evaluate its effect.
Similar content being viewed by others
Introduction
Fluid therapy is a cornerstone of the treatment of hemodynamically unstable critically ill patients [1]. Principal reasons for fluid therapy are correction of hypovolemia and improvement of tissue perfusion. Fluid challenge techniques have replaced static filling pressures as predictors of preload dependency, being used in clinical practice to evaluate the patient’s response to fluids and to avoid pulmonary edema [2]. The generally accepted concept of "fluid responsiveness" is defined at the level of the heart, according to the Frank–Starling principle. A practical limitation of such an approach is that nonresponders also receive fluids, since the test is evaluated after fluid administration. Passive leg raising and functional hemodynamic variables, derived from the assessment of the dynamic interactions between circulation and ventilation, may help to predict "fluid responsiveness", although being hampered by many practical limitations [3, 4]. However, such a macrohemodynamic perspective does not address the fundamental issue of “adequate” organ perfusion. How can one define and select patients with “inadequate” organ perfusion other than by clinical or biochemical signs of organ dysfunction? And does organ perfusion indeed improve in case of macrohemodynamic “fluid responsiveness”?
New microcirculatory imaging techniques, such as orthogonal polarization spectral (OPS) imaging [5] and its technical successor sidestream dark-field (SDF) imaging [6], have allowed direct observation of microcirculation at the bedside. Conceptually such techniques enable us to arbitrate tissue perfusion directly. In animal models of hemorrhagic shock blood loss and fluid therapy was indeed reflected in microcirculatory blood flow [7, 8]. Pottecher et al. [9] showed that both passive leg raising and volume expansion improved sublingual microcirculatory perfusion during the first 24 h of ICU stay in preload-responsive severe sepsis and septic shock patients. Others observed that fluid challenge improved microvascular perfusion in the early but not late phase of severe sepsis and suggested this effect to be independent from systemic hemodynamics and type of fluids [10].
The aim of this study is to address the following questions: (1) If the clinician is confronted with presumed “clinical signs of impaired organ perfusion,” than what is the actual incidence of abnormal organ perfusion when observed with direct in vivo microscopy? (2) How do patients with abnormal organ perfusion at baseline respond to fluid administration in terms of organ perfusion (in vivo microscopy) and organ function (clinical signs)? (3) Is this response different in comparison with patients with normal organ perfusion at baseline? (4) Is an improvement in organ perfusion restricted to patients with a rise in stroke volume (SV) ≥10 % after fluid administration? We anticipated that red blood cell velocity, rather than capillary density, would respond directly to fluid administration; therefore we chose the microvascular flow index (MFI) as the primary variable to quantify organ perfusion.
Patients and methods
Patients
The study was performed between January and March 2011 in a closed-format 22-bed mixed ICU in a tertiary teaching hospital. It was designed as a prospective, single-center, observational clinical trial and is registered with ClinicalTrials.gov with number NCT01369524. All ICU patients of at least 18 years of age with an invasive cardiac output device in situ were eligible for assessment within the first 6 h of ICU admittance. The principal reason for inclusion was the clinical need for fluid therapy, according to one or more predefined signs of impaired organ perfusion: tachycardia >100 beats/min, after exclusion of pain/discomfort; impaired skin perfusion (subjective assessment); oliguria (<0.5 ml/kg/h); mean arterial blood pressure (MAP) <65 mmHg in combination with central/mixed venous oxygen saturation [S(c)vO2] <65 %; hyperlactatemia (>2.2 mmol/l). Reason for exclusion was recent oropharyngeal surgery. A local ethical and scientific committee approved the study protocol, and written informed consent was obtained from the patients or their surrogate decision-makers, consistent with applicable laws.
Protocol
Systemic hemodynamic assessment was achieved through continuous invasive monitoring of arterial blood pressure and right heart catheterization with continuous cardiac output measurements (Vigilance®; Edwards Lifesciences, Saint-Prex, Switzerland). Alternatively, a designated femoral artery thermodilution catheter was used in combination with a central venous line above the diaphragm (PiCCO®; Pulsion Medical Systems, Munich, Germany). Pulse contour analysis of arterial waveforms was performed after calibration with transpulmonary bolus thermodilution. The general aim of the hemodynamic treatment was cessation of the previously defined signs of impaired organ perfusion. In case these endpoints were not met, the first step was to initiate a fluid challenge. This was performed by infusion of 500 ml crystalloid solution (NaCl 0.9 %) or balanced colloid solution (6 % hydroxyethyl starch 130/0.4 in a sodium acetate-based isotonic solution, Volulyte®; Fresenius-Kabi, Bad-Homburg, Germany) in 30 min; the choice of fluid type was made by the attending physician.
Imaging and analysis
Sidestream dark-field imaging is a stroboscopic light-emitting diode ring-based imaging modality that is incorporated in a handheld device [6]. The device illuminates an area of interest for clinical observation of the microcirculation. If a wavelength within the hemoglobin absorption spectrum (e.g., 530 nm) is chosen, red blood cells will appear dark. Semiquantitative analysis was performed as described in detail elsewhere [11]. In short, a minimum of three steady images of at least 10 s in duration were obtained from the sublingual region by a research investigator other than the attending physician. After gentle removal of saliva by an isotonic-saline-drenched gauze and avoiding pressure artifacts, images were acquired and stored on a digital videotape (SONY Video Walkman GV-D 1000E®; Sony, Tokyo, Japan). Subsequently, the images were captured in 5–10 s representative AVI format video clips (SonyDVgate®; Sony, Tokyo, Japan). Video clips were blindly analyzed offline by an investigator who had no involvement in the data collection. The images were presented in random order so as to prevent interimage coupling. SDF images were obtained from three different locations within the sublingual region, and each image was divided into four equal quadrants. Quantification of flow (no flow: 0; intermittent flow: 1; sluggish flow: 2; continuous flow: 3) was scored per quadrant, for each vessel diameter cohort (small: 10–20 μm; medium: 21–50 μm; large: 51–100 μm). The MFI was calculated as the sum of each quadrant score divided by the number of quadrants in which the vessel type was visible. The final MFI was averaged over a maximum of 12 quadrants (three regions, four quadrants per region) derived from the overall flow impressions of all vessels with a particular range of diameter in a given quadrant. The heterogeneity index was calculated, following the method of Trzeciak and colleagues [12], as the difference between the highest and lowest MFI, divided by the mean MFI of all sublingual sites at a single time point. Calculation of total (small) vessel density (TVD) was performed with the AVA 3.0® software package (MicroVision Medical, Amsterdam, The Netherlands), as described and validated recently [13], using a cutoff diameter for small vessels of <20 μm. After stabilization of the images using the AVA 3.0 software, we defined the perfused (small) vessel density (PVD) and the proportion of perfused (small) vessels (PPV) in terms of the number and percentage of crossings with perfused (small) vessels per total length of three equidistant horizontal and three equidistant vertical lines. This method has been described elsewhere by de Backer et al. and is in accordance with reports of a round-table conference [14].
Data collection
The following data were recorded at baseline: general characteristics, routine laboratory results, ventilator settings, and severity of illness according to Acute Physiology and Chronic Health Evaluation (APACHE) IV and Sequential Organ Failure Assessment (SOFA) scores, calculated over the first 24 h following ICU admission [15, 16]. Systemic hemodynamic variables, SDF images, arterial lactate concentrations, and blood gases were recorded at baseline and directly after the fluid challenge (30 min after baseline). At baseline, patient groups were defined according to MFI <2.6 (“low MFI”) or MFI ≥2.6 (“high MFI”). The primary endpoint was the percentage of patients with MFI <2.6 at baseline. The cutoff for MFI was set as 2.6 prior to the start of the study (ClinicalTrials.gov, NTC 00493415), since this reflects the minimum reported lower threshold of the 95 % confidence interval in healthy or nonseptic ICU controls [12, 17–20]. Secondary endpoints were the changes in MFI, “clinical signs of impaired organ perfusion,” and SV after fluid administration, and the differences in response between groups.
Statistical analysis
Baseline data for a power calculation were not available. Alternatively, a study period of 3 months was chosen to obtain a sample size of 30–50 patients, in analogy to previous publications [7, 9, 10]. The Statistical Package for Social Sciences (SPSS 15.1 for Windows, Chicago, IL, USA) was used for statistical analyses. For continuous variables, all data are presented as median and interquartile range (IQR). Comparison between groups of non-normally distributed microvascular parameters (MFI, TVD, PPV, PVD) was performed with a Mann–Whitney U test. Comparison against baseline of systemic and microcirculatory hemodynamic parameters after intervention was performed with a paired t test, or with a Wilcoxon signed-rank test in case of normally distributed variables. For dichotomous variables a Fisher’s exact test was performed. A two-sided p value of <0.05 was considered statistically significant.
Results
Out of 57 patients who were eligible for the study, 50 patients were included; in seven cases informed consent could not be obtained. Baseline characteristics are presented in Table 1. Sixty-six percent (33/50) of the patients had MFI <2.6 at baseline. The median number of “clinical signs of impaired organ perfusion” was 1 (1–2). Post hoc, a receiver operating characteristic (ROC) curve confirmed a MFI of 2.6 as the optimal cutoff value with respect to the clinical response to fluid administration.
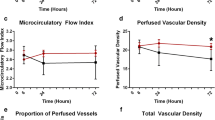
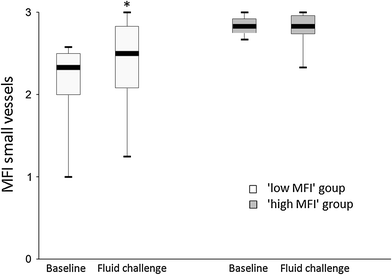
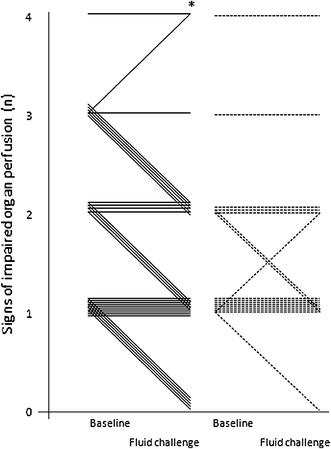
Low MFI group (MFI <2.6 at baseline, n = 33): After fluid challenge central venous pressure (CVP), SV index (SVi), and S(c)VO2 rose significantly, together with a reduction in heart rate (Table 2). Median MFI of small vessels, as a marker of capillary blood flow, increased significantly from 2.3 (2–2.5) at baseline to 2.5 (2.1–2.8) after fluid challenge (p = 0.003) (Table 3; Fig. 1). In conjunction with the rise in MFI the median number of “clinical signs of impaired organ perfusion” reduced from 2 (1–2) to 1 (0–2) (p < 0.001) (Fig. 2). The number of patients with a reduction in number of “clinical signs of impaired organ perfusion” after fluid challenge was 15 (45 %). TVD of small vessels, as a marker of capillary density, did not change significantly from 30 (27–34) mm/mm2 at baseline to 31 (26–36) mm/mm2 (p = 0.48).
High MFI group (MFI ≥2.6 at baseline, n = 17): After fluid challenge SVi rose significantly, together with a reduction in heart rate (Table 2). However, median MFI of small vessels remained unaltered: 2.8 (2.8–2.9) at baseline versus 2.8 (2.7–3) (p = 0.45) (Table 3; Fig. 1). Also, the number of “clinical signs of impaired organ perfusion” did not change significantly, from 1 (1–2) at baseline versus 1 (1–2) after fluid challenge (p < 0.32) (Fig. 2). The number of patient with a reduction in number of “clinical signs of impaired organ perfusion” after fluid challenge was 3 (18 %). In comparison with the low MFI group this reduction was significantly lower (45 vs. 18 %, p = 0.05). TVD of small vessels did not change significantly: from 27 (25–32) mm/mm2 at baseline, to 28 (23–32) mm/mm2 (p = 0.72).
Responders versus nonresponders (ΔSVi ≥10 % vs. ΔSVi <10 %): In 68 % of all patients SVi increased ≥10 % after fluid challenge (Table 2). There was no difference in the number of responders between the low MFI and high MFI groups: 23/33 (70 %) versus 11/17 (65 %) (p = 0.78). In the responders (N = 34), MFI of small vessels rose significantly from 2.5 (2.3–2.8) at baseline to 2.7 (2.4–2.8) after fluid administration (p = 0.02). The median number of “clinical signs of impaired organ perfusion” reduced from 1.5 (1–2) to 1 (1–2) (p = 0.01). In the nonresponders (N = 16), MFI of small vessels also rose from 2.5 (2.2–2.9) to 2.7 (2.4–2.9), but this was insignificant (p = 0.09). The median number of “clinical signs of impaired organ perfusion” reduced from 2 (1–3) to 1.5 (1–2) (p = 0.01).
Discussion
In the present study, sublingual microvascular flow alterations, defined as MFI <2.6, were present in two-thirds of mixed ICU patients with presumed “clinical signs of impaired organ perfusion.” Post hoc ROC analysis confirmed MFI <2.6 as the optimal cutoff value. This indicates a rather low specificity of widely used clinical criteria that intensive care doctors assume to be indicators of impaired organ perfusion. In the low MFI group, fluid administration was accompanied by significant attenuation of impaired microvascular blood flow and reduction of “clinical signs of impaired organ perfusion.” However, in the high MFI group, fluid administration was associated with neither a change in microvascular blood flow nor mitigation of these clinical signs, suggesting alternative causes for such symptoms in these patients. Assessment of microcirculatory flow abnormalities, prior to fluid therapy, may therefore be helpful to increase the pre-test likelihood of such intervention. The data from this study underline the importance of selecting patients eligible for fluid therapy and monitoring the effects. Since none of the macrohemodynamic variables at baseline discriminated for MFI <2.6, it seems relevant to assess organ perfusion at the level of the microcirculation. The fact that scoring of MFI has been demonstrated to be feasible in real time at the bedside adds to its clinical applicability for selecting patients and monitoring therapeutic interventions [21]. As shown in our data, it is of note to use variables of flow (MFI or PPV) rather than variables that represent capillary distance (PVD, TVD).
Apart from our newly suggested approach to select patients prior to fluid therapy, our data seem to add to the existing literature in other perspectives as well. Previous publications linked (the persistence of) rather gross microvascular alterations to organ dysfunction, severity of illness, and prognosis [12, 17, 22–24]. In these studies, with relatively small sample sizes, strict selection of specific subgroups of patients, with profound abnormalities at baseline, are helpful to detect potential mechanisms. However, such studies do not mimic clinical reality to the full extent. Clinical decision-making in the ICU is often characterized by uncertainties regarding whether to intervene in relatively mild abnormalities in a heterogeneous population. In our study, median MFI was “only” 2.5 and median PPV 90 %, with a lowest 25th percentile at 2.3 and 86 %, respectively. These data add to the understanding that even relatively mild microvascular flow alterations are associated with clinical signs of impaired organ perfusion, and may be mitigated by therapy, before transition to prolonged organ dysfunction [20, 25]. Furthermore, our data seem to expand the existing knowledge of microvascular abnormalities and response to fluid therapy, from predominantly sepsis patients [9, 10] to a more heterogeneous ICU population.
The relationship between the commonly used concept of “fluid responsiveness” (i.e., ΔSVi ≥10 % in response to a fluid challenge) and the mitigation of microcirculatory abnormalities seems to be rather complex. At baseline, the static dichotomous variable responder/nonresponder discriminated neither between MFI <2.6 or ≥2.6 nor between the presence or absence of “clinical signs of impaired organ perfusion.” This highlights the issue that a significant increase in SV after fluid administration is not equal to the need for fluid therapy. In daily practice not only is “fluid responsiveness” difficult to predict [26], but means (“responsiveness”) and objective (improvement of organ perfusion) are also often mistaken. Moreover, the absolute increase in MFI was equal in patients with and without an increase in SVi ≥10 %. Our observations suggest that, within the 30-min time window of the fluid challenge, microcirculatory flow alterations may improve, independent from the change in SV. This seems to be in contradiction to the idea that a systemic enhancement of flow (e.g., cardiac output) is a prerequisite for an augmentation of organ perfusion at the level of the microcirculation.
These data seem to be in line with the existing literature. In preload-dependent septic patients, with marked microvascular alterations at baseline, the effect of fluid therapy was reflected both at the level of the heart and in the microcirculation [9]. However, in this study nonresponders and nonseptic patients were not included. Others observed a time-dependent relation between fluid administration and microvascular response in septic patients, irrespective of (a change in) blood pressure or cardiac output [10]. This might be due to differences in the pathophysiology of microvascular alterations over time. However, the fact that, in this study, microvascular variables were considerable higher in the late phase of sepsis than in the early phase also underpins the importance of establishing the magnitude of potentially correctable abnormalities prior to intervention.
Several limitations of the study need to be addressed. Firstly, this was an uncontrolled study. Although the observation period was short (30 min), spontaneous improvement in microvascular blood flow cannot be ruled out. However, as of now, denial of fluids is considered unethical under these circumstances. Secondly, the absence of effect on clinical signs of organ perfusion may have been hampered by the chosen timeframe and amount/type of fluid administered. Clinical signs may have needed longer time to respond to the intervention, and in individual patients more fluids may have been necessary to detect effects. The fact that both crystalloid and colloid solutions have been used is of potential concern. Some authors observed marked differences in microvascular response after 24 h in sepsis with either isotonic saline or HES 6 % 130/0.4 [27], whereas others could not observe such difference between Ringer’s lactate and 4 % albumin [10], or colloids and hypertonic saline [7, 28]. In a post hoc analysis we were unable to demonstrate significant differences in SV and MFI in response to different types of fluids (Electronic Supplementary Material). Thirdly, the definitions of impaired organ perfusion were chosen in line with a local protocol, rather than a more standardized SOFA score. Although small but significant changes in SOFA score have been described to be associated with microvascular alterations during protocol-directed resuscitation [25], we considered large parts of the SOFA score potentially insensitive to detect changes in organ perfusion. Lastly, we used the tongue as a window to observe the microcirculation. It is conceivable that other organs may express different microvascular abnormalities at baseline [18, 29], or in response to fluid administration [30].
Conclusions
In the present study microvascular flow abnormalities, defined as MFI <2.6, were present in two-thirds of hemodynamically unstable ICU patients with “clinical signs of impaired organ perfusion.” In response to fluid administration, a significant increase in microvascular blood flow and attenuation of “clinical signs of impaired organ perfusion” were restricted to patients with MFI <2.6 at baseline. This response was irrespective of a change in SV. These data add to the understanding that noninvasive assessment of microvascular perfusion may help to identify patients with potential need for fluid therapy, and to evaluate its effect.
References
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL (2008) Surviving sepsis campaign: International Guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med 34:17–60. doi:10.1007/s00134-007-0934-2
Vincent JL, Weil MH (2006) Fluid challenge revisited. Crit Care Med 34:1333–1337. doi:10.1097/01.CCM.0000214677.76535.A5
Vincent JL, Rhodes A, Perel A, Martin GS, Della Rocca G, Vallet B, Pinsky MR, Hofer CK, Teboul JL, de Boode WP, Scolletta S, Vieillard-Baron A, De Backer D, Walley KR, Maggiorini M, Singer M (2011) Clinical review: update on hemodynamic monitoring—a consensus of 16. Crit Care 15:229. doi:10.1186/cc10291
Michard F, Teboul JL (2002) Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 121:2000–2008
Groner W, Winkelman JW, Harris AG, Ince C, Bouma GJ, Messmer K, Nadeau RG (1999) Orthogonal polarization spectral imaging: a new method for study of the microcirculation. Nat Med 5:1209–1212. doi:10.1038/13529
Goedhart PT, Khalilzada M, Bezemer R, Merza J, Ince C (2007) Sidestream dark field (SDF) imaging: a novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Opt Express 15:15101–15114
Maier S, Holz-Hölzl C, Pajk W, Ulmer H, Hengl C, Dünser M, Haas T, Velik-Salchner C, Fries D, Greiner A, Hasibeder W, Knotzer H (2009) Microcirculatory parameters after isotonic and hypertonic colloidal fluid resuscitation in acute hemorrhagic shock. J Trauma 66:337–345. doi:10.1097/TA.0b013e31817dac66
Dubin A, Pozo MO, Ferrara G, Murias G, Martins E, Canullán C, Canales HS, Kanoore Edul VS, Estenssoro E, Ince C (2009) Systemic and microcirculatory responses to progressive hemorrhage. Intensive Care Med 35:556–564. doi:10.1007/s00134-008-1385-0
Pottecher J, Deruddre S, Teboul JL, Georger JF, Laplace C, Benhamou D, Vicaut E, Duranteau J (2010) Both passive leg raising and intravascular volume expansion improve sublingual microcirculatory perfusion in severe sepsis and septic shock patients. Intensive Care Med 36:1867–1874. doi:10.1007/s00134-010-1966-6
Ospina-Tascon G, Neves AP, Occhipinti G, Donadello K, Büchele G, Simion D, Chierego ML, Silva TO, Fonseca A, Vincent JL, De Backer D (2010) Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med 36:949–955
Boerma EC, Mathura KR, van der Voort PHJ, Spronk PE, Ince C (2005) Quantifying bedside-derived imaging of microcirculatory abnormalities in septic patients: a prospective validation study. Crit Care 9:R601–R606. doi:10.1186/cc3809
Trzeciak S, Dellinger RP, Parrillo JE, Guglielmi M, Bajaj J, Abate NL, Arnold RC, Colilla S, Zanotti S, Hollenberg SM (2007) Microcirculatory alterations in resuscitation and shock investigators. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med 49:88–98. doi:10.1016/j.annemergmed.2006.08.021 98.e1
Dobbe JGG, Streekstra GJ, Atasever B, van Zijderveld R, Ince C (2008) Measurement of functional microcirculatory geometry and velocity distributions using automated image analysis. Med Biol Eng Comput 46:659–670. doi:10.1007/s11517-008-0349-4
De Backer D, Hollenberg S, Boerma C, Goedhart P, Büchele G, Ospina-Tascon G, Dobbe I, Ince C (2007) How to evaluate the microcirculation: report of a round table conference. Crit Care 11:R101. doi:10.1186/cc6118
Zimmerman JE, Kramer AA, McNair DS, Malila FM (2006) Acute physiology and chronic health evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med 34:1297–1310. doi:10.1097/01.CCM.0000215112.84523.F0
Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S (1998) Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of intensive care medicine. Crit Care Med 26:1793–1800
Spanos A, Jhanji S, Vivian-Smith A, Harris T, Pearse RM (2009) Early microvascular changes in sepsis and severe sepsis. Shock. doi:10.1097/SHK.0b013e3181c6be04
Boerma EC, van der Voort PHJ, Spronk PE, Ince C (2007) Relationship between sublingual and intestinal microcirculatory perfusion in patients with abdominal sepsis. Crit Care Med 35:1055–1060. doi:10.1097/01.CCM.0000259527.89927.F9
Edul VSK, Enrico C, Laviolle B, Vazquez AR, Ince C, Dubin A (2012) Quantitative assessment of the microcirculation in healthy volunteers and in patients with septic shock. Crit Care Med. doi:10.1097/CCM.0b013e31823dae59
Jhanji S, Lee C, Watson D, Hinds C, Pearse RM (2009) Microvascular flow and tissue oxygenation after major abdominal surgery: association with post-operative complications. Intensive Care Med 35:671–677. doi:10.1007/s00134-008-1325-z
Arnold RC, Parrillo JE, Phillip Dellinger R, Chansky ME, Shapiro NI, Lundy DJ, Trzeciak S, Hollenberg SM (2009) Point-of-care assessment of microvascular blood flow in critically ill patients. Intensive Care Med 35:1761–1766. doi:10.1007/s00134-009-1517-1
De Backer D, Creteur J, Dubois MJ, Sakr Y, Vincent JL (2004) Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J 147:91–99
Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL (2004) Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 32:1825–1831
Sheikh MY, Javed U, Singh J, Choudhury J, Deen O, Dhah K, Peterson MW (2009) Bedside sublingual video imaging of microcirculation in assessing bacterial infection in cirrhosis. Dig Dis Sci. doi:10.1007/s10620-008-0664-5
Trzeciak S, McCoy JV, Phillip Dellinger R, Arnold RC, Rizzuto M, Abate NL, Shapiro NI, Parrillo JE, Hollenberg SM (2008) Microcirculatory alterations in resuscitation and shock (MARS) investigators Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive Care Med 34:2210–2217. doi:10.1007/s00134-008-1193-6
Lansdorp B, Lemson J, van Putten MJAM, de Keijzer A, van der Hoeven JG, Pickkers P (2012) Dynamic indices do not predict volume responsiveness in routine clinical practice. Br J Anaesth 108:395–401. doi:10.1093/bja/aer411
Dubin A, Pozo MO, Casabella CA, Murias G, Pálizas F, Moseinco MC, Kanoore Edul VS, Estenssoro E, Ince C (2010) Comparison of 6 % hydroxyethyl starch 130/0.4 and saline solution for resuscitation of the microcirculation during the early goal-directed therapy of septic patients. J Crit Care 25(4):659.e1–659.e8. doi:10.1016/j.jcrc.2010.04.007
van Haren FMP, Sleigh J, Boerma EC, La Pine M, Bahr M, Pickkers P, van der Hoeven JG (2012) Hypertonic fluid administration in patients with septic shock: a prospective randomized controlled pilot study. Shock 37:268–275. doi:10.1097/SHK.0b013e31823f152f
Pranskunas A, Pilvinis V, Dambrauskas Z, Rasimaviciute R, Planciuniene R, Dobozinskas P, Veikutis V, Vaitkaitis D, Boerma EC (2012) Early course of microcirculatory perfusion in eye and digestive tract during hypodynamic sepsis. Crit Care 16:R83. doi:10.1186/cc11341
Dubin A, Edul VS, Pozo MO, Murias G, Canullán CM, Martins EF, Ferrara G, Canales HS, Laporte M, Estenssoro E, Ince C (2008) Persistent villi hypo-perfusion explains intra-mucosal acidosis in sheep endotoxemia. Crit Care Med 36:535–542. doi:10.1097/01.CCM.0000300083.74726.43
Acknowledgments
A local hospital scientific committee provided an unrestricted grant in support of this study.
Conflicts of interest
The authors state that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
134_2012_2793_MOESM1_ESM.tif
Supplementary Figure 1 Flowchart of patient inclusion according to baseline microvascular flow index (MFI) and type of fluid (TIFF 58 kb)
134_2012_2793_MOESM2_ESM.tif
Supplementary Figure 2 Boxplots of the microvascular flow index (MFI) in response to fluid administration in different types of fluids. * p < 0.05 (TIFF 94 kb)
134_2012_2793_MOESM3_ESM.tif
Supplementary Figure 3 Boxplots of the stroke volume index in response to fluid administration in different types of fluids. * p < 0.05 (TIFF 92 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Pranskunas, A., Koopmans, M., Koetsier, P.M. et al. Microcirculatory blood flow as a tool to select ICU patients eligible for fluid therapy. Intensive Care Med 39, 612–619 (2013). https://doi.org/10.1007/s00134-012-2793-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2793-8