Abstract
Purpose
The recent increase in drug-resistant micro-organisms complicates the management of hospital-acquired bloodstream infections (HA-BSIs). We investigated the epidemiology of HA-BSI and evaluated the impact of drug resistance on outcomes of critically ill patients, controlling for patient characteristics and infection management.
Methods
A prospective, multicentre non-representative cohort study was conducted in 162 intensive care units (ICUs) in 24 countries.
Results
We included 1,156 patients [mean ± standard deviation (SD) age, 59.5 ± 17.7 years; 65 % males; mean ± SD Simplified Acute Physiology Score (SAPS) II score, 50 ± 17] with HA-BSIs, of which 76 % were ICU-acquired. Median time to diagnosis was 14 [interquartile range (IQR), 7–26] days after hospital admission. Polymicrobial infections accounted for 12 % of cases. Among monomicrobial infections, 58.3 % were gram-negative, 32.8 % gram-positive, 7.8 % fungal and 1.2 % due to strict anaerobes. Overall, 629 (47.8 %) isolates were multidrug-resistant (MDR), including 270 (20.5 %) extensively resistant (XDR), and 5 (0.4 %) pan-drug-resistant (PDR). Micro-organism distribution and MDR occurrence varied significantly (p < 0.001) by country. The 28-day all-cause fatality rate was 36 %. In the multivariable model including micro-organism, patient and centre variables, independent predictors of 28-day mortality included MDR isolate [odds ratio (OR), 1.49; 95 % confidence interval (95 %CI), 1.07–2.06], uncontrolled infection source (OR, 5.86; 95 %CI, 2.5–13.9) and timing to adequate treatment (before day 6 since blood culture collection versus never, OR, 0.38; 95 %CI, 0.23–0.63; since day 6 versus never, OR, 0.20; 95 %CI, 0.08–0.47).
Conclusions
MDR and XDR bacteria (especially gram-negative) are common in HA-BSIs in critically ill patients and are associated with increased 28-day mortality. Intensified efforts to prevent HA-BSIs and to optimize their management through adequate source control and antibiotic therapy are needed to improve outcomes.
Similar content being viewed by others
Introduction
Bloodstream infection (BSI) is an important cause of severe sepsis and septic shock that is associated with high resource utilisation, morbidity and mortality [1–4]. Hospital-acquired bloodstream infection (HA-BSI) is recognised as a major patient-safety concern and a marker of quality of care [5]. Patients admitted to intensive care units (ICUs) have multiple risk factors for HA-BSIs including severe acute illness, co-morbidities and frequent use of invasive devices [6]. Many changes have occurred in the epidemiology of BSI in recent years, particularly with the emergence of drug-resistant organisms, which has increased the treatment-failure rate and the risk of adverse patient outcomes [2, 6]. Although hospital-acquired infections in critically ill patients have been the focus of numerous reports worldwide [2–8], there is a paucity of contemporary multinational data on the epidemiology and outcome determinants of HA-BSIs in ICU patients [2, 4, 9].
The objective of this study is to describe the epidemiology of HA-BSIs treated in the ICU and to identify determinants of treatment failure and outcomes in Europe and internationally.
Methods
This study is reported in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines [10].
A prospective observational multicentre international cohort design was used. All participating ICUs obtained approval from their local ethics committees. The pre-defined goal was to include 1,100 ICU patients with HA-BSI.
Study protocol and definitions
Patients were enrolled if they had a new diagnosis of HA-BSI and were admitted to an ICU. The study focussed on the first episode of HA-BSI. Detailed definitions of HA-BSI are provided in the Electronic Supplement.
Data collected for each patient included the dates and times of collection and positivity of the first positive blood culture; source of infection; presence of sepsis; severity of illness; co-morbidities; and management including source control, antimicrobial drugs and adjunctive treatments. All study data were obtained from patient files, and no additional tests were performed for the purpose of the study. Severity of illness was defined at ICU admission and at HA-BSI diagnosis using SAPS II and Sequential organ failure assessment (SOFA) scores [11], respectively. Co-morbidities were assessed using the Charlson index and the five markers of the Chronic Health Evaluation of the APACHE II score reported by Knaus et al. [12, 13].
Clinical variables and relapses or new episodes of HA-BSI were recorded until ICU discharge, and all-cause mortality within 28 days since first positive blood culture was ascertained.
We recorded information on each ICU including type of hospital and ICU, number of beds and patients, and mortality rate in the previous year (2008). We also recorded factors possibly associated with antimicrobial use such as the availability of an infectious diseases specialist, procedures for antibiotic use and infection control protocols.
The organisms causing HA-BSI and their antimicrobial susceptibility test results were recorded according to local policies. Detailed definitions of adequate antimicrobial therapy are given in the Electronic Supplement.
Drug-resistant organisms were classified as susceptible (SUS), multidrug resistant (MDR), extensively resistant (XDR) and pan-drug resistant (PDR) according to the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) [14]. Each category was included within the previous category: all PDR organisms were XDR, and all XDR organisms were MDR. Definitions and worksheets can be downloaded from the European Centre for Disease Prevention and Control (ECDC) website [15]. According to this classification scheme, bacteria other than enterococci, Staphylococcus aureus, Enterobacteriaceae and non-fermenting gram-negative bacteria were considered susceptible. We arbitrarily classified yeasts among MDR organisms on the basis of their non-sensitivity to first-line empirical antimicrobial therapies.
Data management and statistical analysis
In each study ICU, data were entered into a password-protected and secured web-based server. Online data were managed in MySQL 5.1.41 (Oracle, Redwood Shores, CA, USA) and downloaded to SAS 9.2 (SAS Institute, Cary, NC, USA) for offline management and analysis. The electronic case-report form was developed locally using open-source software (PHP/MySQL) with the primary goal of ensuring easy and consistent data entry, data verification and easy communication between the coordinating centre (Albert Bonniot Institute, University Joseph Fourier, Grenoble, France) and each study ICU. At the coordinating centre, two investigators (A.T., D.K.) routinely checked the data for completeness and for consistency in definition use. All missing, extreme or implausible values were sent back to the study-ICU investigators for review. Where data could not be confirmed or remained questionable, the primary author (A.T.) made a final adjudication about study inclusion, in agreement with the main investigator (J.-F.T.). Doubtful cases were reviewed by two other investigators (J.-R.Z. and K.L.). Missing data were replaced by the median value, and missing times of sampling and of blood culture positivity by 12:00 a.m.
The statistical analyses considered only the first episode of HA-BSI, as information was fully collected only for these occurrences.
Means with standard deviation (SD) were used to describe normally or near-normally distributed continuous data and were compared using the t test. Medians with interquartile range (IQR) were computed for skewed data and were compared using the Mann–Whitney test. Fisher’s exact test or the chi-square test was performed to compare categorical data.
In multivariable analyses, variables were organised into three tiers: country, ICU and patient. To identify factors associated with 28-day mortality, we built a three-tiered hierarchical logistic mixed model using the GLIMMIX procedure of the SAS software. The influence of country-based and ICU-based variables on the outcome was included through both fixed and random effects. Multilevel modelling takes into account the hierarchical structure of the data, which may manifest as intra-class correlations. To obtain a conservative estimate of the standard error, a separate random-error term should be specified for each level of the analysis [16]. Therefore, to avoid overestimating the significance of risk factors for death by day 28, we took intra-class correlations into account, and we specified a separate random-error term for each tier. Variables potentially associated with 28-day mortality (p values less than 0.10 on univariate analysis) were introduced into the multivariable model and selected using a backward approach. Two-way clinically relevant interactions were tested in the final model. In all analyses, two-sided p values less than 0.05 were deemed statistically significant. No correction for multiple testing was performed.
Results
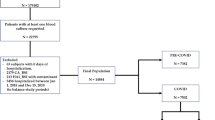
We enrolled 1,156 patients from 162 ICUs in 120 cities in 24 countries (Fig. 1). Sixty-three ICUs accepted the study but did not participate, and 16 ICUs (30 patients) were excluded because of incomplete data. Of the 1,227 patients included initially, 71 were excluded prior to data analysis (34 incomplete files, 8 patients with community-acquired BSI and 29 patients with cultures positive for skin contaminants), leaving 1,156 patients for the study.
The study patients were located in many different geographic regions, although they predominated in European countries, particularly France (17.8 %) and Greece (24.9 %) (Fig. 1). No country-related factors were significantly associated with 28-day mortality (Table E1, Electronic Supplement).
Most of the ICUs (64 %) were university or university-affiliated. An infectious disease specialist was available in 86 % of the ICUs. Written antibiotic procedures were available in 71 % of ICUs and followed strictly in 23 %. Further details on ICU-related prognosis factors are presented in Table 1.
A total of 1,317 bloodstream isolates were cultured from the 1,156 study patients. A single organism was found in 1,016 (88 %) patients, two organisms in 120 (10 %) patients, three organisms in 19 (2 %) patients and four organisms in 1 (<1 %) patient.
HA-BSIs were diagnosed a median of 14 (IQR, 7–26) days after hospital admission. Among them, 76 % (877/1,156) were diagnosed in the ICU, with a median time from ICU admission to diagnosis of 8 (IQR, 3–16) days. Timing of acquisition had no significant influence on mortality. Patient characteristics are reported in Table 2 and details on the infections and treatment in Table 3.
The most common organisms causing HA-BSI and their resistance patterns are presented in Table 4. Of monomicrobial infections (n = 1,016), 592 (58.3 %) were gram-negative, 333 (32.8 %) gram-positive, 79 (7.8 %) fungal and 12 (1.2 %) due to strict anaerobes.
Carbapenem resistance was found in 110/166 (69 %) Acinetobacter spp., 59/156 (38 %) Klebsiella pneumoniae, 56/150 (37 %) Pseudomonas spp., 5/88 (5.7 %) Enterobacter spp. and 1/98 (1 %) Escherichia coli. Of the 119 S. aureus isolates, 57 (48 %) were methicillin-resistant; and of the 70 Enterococcus faecium isolates, 16 (23 %) were vancomycin-resistant (VRE). Isolates acquired in the ICU were more often drug resistant compared with other hospital-acquired isolates (413/992, 42 % versus 82/325, 25 %; p < 0.0001). The distribution of organisms differed significantly (p < 0.001) by country (Table E2, Electronic Supplement).
Among the 1,156 study patients, 608 (52.6 %) received adequate antibiotic therapy before or within 24 h following collection of the first positive blood culture, whereas 154 (13.3 %) did not receive adequate antibiotic therapy within 5 days or before ICU discharge or death. Patients who received adequate antimicrobials only after the fifth day had lower SOFA scores. Late adequate therapy was also associated with a longer time to blood-culture positivity (median [IQR], 100 h [23–144]). Resistance rates showed a significant positive association with failure to receive adequate antibiotic therapy. As shown in Fig. 2, time to adequate antibiotic therapy increased with antimicrobial resistance (chi-square for trends, p < 0.001).
Cumulative percentage of patients receiving at least one adequate antimicrobial, on each calendar day before and after the date of collection of the first positive blood culture, shown by antimicrobial resistance status (trend chi-square, p < 0.001). The calendar day (without details on time) was available in 46 patients for blood collection and 67 patients for treatment initiation; a time of 12:00 a.m. was assigned in these patients
The most frequently prescribed empirical antimicrobials and their adequacy against the causative organism are shown in Fig. 3a, and the first adequate treatment is shown in Fig. 3b. Carbapenems were the antimicrobials most frequently given in the first 24 h (n = 212; 19 %), followed by glycopeptides (n = 172; 15 %) and piperacillin-tazobactam (n = 152; 13 %). Treatment was adequate in the first 24 h with monotherapy in 339 (29 %) patients and combined therapy in 269 (23 %) patients. The 548 (47 %) remaining patients did not receive adequate antimicrobials in the first 24 h.
Antibiotic use. a Most frequent empiric antimicrobials, reported as the number of patients receiving the antimicrobial in the 12 h before, to 24 h after collection of the first positive blood culture. b Antimicrobials most frequently used in the 5 days following HA-BSI diagnosis, reported as the number of patients receiving the treatment. “Adequate” and “inadequate” show the percentage of cases in which the organism recovered from blood cultures was susceptible to each molecule
The 28-day all-cause mortality rate was 413/1,156 (35.7 %), with 381/413 (92 %) deaths occurring in the ICU. On day 28, 295/743 (40 %) survivors were still in the ICU. Among the 1,156 patients, 194 (17 %) had second and 44 (4 %) had third episodes of HA-BSI identified within the 28-day follow-up period. Patient- and ICU-related factors included in the univariate analysis are presented in Tables 1–3.
On multivariable analysis (Table 5), factors significantly associated with higher 28-day mortality were turn-over above the median value, higher mean ICU mortality rate in the previous year (2008) and several patient-related factors (Table 5). 28-day mortality was not significantly influenced by factors that may reflect antimicrobial stewardship (availability of an infectious diseases specialist, microbiological laboratory, written antibiotic protocols and/or infection-control protocols). 28-day mortality was significantly higher in older patients and in patients with chronic respiratory disease or immune deficiency. Intensity of the host response and organ dysfunctions as reflected by septic shock or a higher SOFA score at HA-BSI onset was an independent risk factor for 28-day mortality. The source of infection significantly affected 28-day mortality when introduced as a multiple-class variable (catheter, intra-abdominal, respiratory, urinary, multiple and other focus) (p = 0.02, data not shown). Only abdominal source of infection was associated with 28-day mortality at the final step of variable selection. 28-day mortality was significantly higher in the event of inadequate antibiotic therapy or failure to control the infection source if required (absence of catheter removal, n = 12; surgical treatment for abdominal source of infection, n = 22; other sources, n = 6; multiple possible foci, n = 5).
Presence of an MDR/XDR/PDR organism was associated with a longer time to adequate antimicrobial therapy and with an increase in 28-day mortality. XDR or PDR resistance levels were not associated with higher 28-day mortality when compared with MDR levels. The results remained similar after exclusion of fungal HA-BSIs from the analysis (Table E3, Electronic Supplement) and when considering patients with a single episode of HA-BSI (Table E4, Electronic Supplement). The impact of inadequate antimicrobial treatment was not significantly different between episodes with and without MDR strains (p = 0.54).
Discussion
EUROBACT provides a contemporary analysis of the prognostic factors of HA-BSI among patients admitted to ICUs internationally. There was a predominance of patients included in France, Greece and southern Europe. Drug resistance rates were very high overall, and MDR was particularly common among gram-negative pathogens. Piperacillin + tazobactam, carbapenems, and glycopeptides were extensively used both as initial empirical drugs and once the culture results were available. Mortality was higher in ICUs with higher turn-over rates. Inadequate antibiotic treatment and failure to control the source of infection were both associated with 28-day mortality, independently from age, chronic co-morbidities, severity of acute illness, shock and organ dysfunctions. Antimicrobial resistance was associated with a significantly longer time to adequate antimicrobial treatment and with a higher risk of death, even after controlling for adequacy of antimicrobial treatment.
The epidemiology of BSIs in ICU patients has changed over time. Gram-positive bacteria and yeasts have become major causes of BSI in the last two decades [1, 17]. MDR gram-negative bacteria are re-emerging [18], as confirmed by the present report. The most frequent pathogens in EUROBACT were Acinetobacter, Klebsiella and Pseudomonas spp., followed by enterococci, coagulase-negative staphylococci and S. aureus. The high prevalence of Acinetobacter in our study is probably related to the overrepresentation of southern European countries and may not reflect the situation worldwide. We observed significant variability in the distributions of organism groups and resistance patterns across countries, in accordance with European surveillance reports [15]. MDR organisms were ubiquitous, and three-quarters of the countries reported at least one XDR organism. These data are consistent with earlier reports [15]. Thus, XDR Acinetobacter baumanii and P. aeruginosa and carbapenemase-producing K. pneumoniae have been reported in southern Europe, as well as in South America and Asia, and have shown a tendency to spread rapidly throughout the world [18]. Moreover, with the expansion of international travel [19], no country is exempt from the risk of a major XDR outbreak.
The relationship between promptness of adequate therapy and prognosis is complex. Physicians prescribe early extended-spectrum antimicrobial therapy to patients with severe clinical presentations. In contrast, antimicrobial therapy is often delayed in patients without organ dysfunctions or with long times to blood-culture positivity. Finally, patients who die very early never receive antimicrobial therapy. These considerations explain why treatment adequacy was unrelated to outcomes in recent cohort studies [9, 20, 21]. Observational studies cannot provide proof of a causal relationship between treatment adequacy and outcome. In the present study, we found an association between time to adequate treatment and mortality even after adjustment for acute-illness severity at BSI onset. Further multistate models taking into account adequate antimicrobial therapy as a non-absorbing state and severity of the BSI are needed to definitely estimate the impact of delayed therapy. Although fluid resuscitation and adequate treatment of organ dysfunctions are key components of critical care, prompt adequate antimicrobial treatment remains a cornerstone of BSI management in the ICU [22].
Many studies, including the present one, have found that bacterial resistance decreased the chance of early adequate therapy [23, 24]. Bacterial resistance was also associated with mortality in recent, large, well-conducted epidemiological studies [2, 25]. However, when both bacterial resistance and adequacy of treatment are taken into account, treatment inadequacy is seen to make a larger contribution to the mortality increase than bacterial resistance [9, 26, 27]. One major finding from our study is the significant impact of MDR infections on patient outcome, even after adjustment on antimicrobial treatment adequacy, source control and all other ICU- and patient-related determinants of 28-day mortality. Interestingly, XDR infection did not have a greater influence on 28-day mortality than MDR infection, despite a further increase in time to adequate therapy. This finding is consistent with experimental studies suggesting that resistance to antimicrobial agents may be associated with decreases in bacterial fitness, metabolic activity [28] or virulence [29].
The strong relationship between absence of source control and mortality in our study further supports the widely recognised importance of source control in patients with infection or sepsis [22]. To the best of our knowledge, although this importance is widely accepted by clinicians, it has not been demonstrated in previous studies, except in necrotising fasciitis [30].
In the present study, an intra-abdominal source was an independent risk factor for mortality, in keeping with previously reported evidence that intra-abdominal sources of bacteraemia were associated with higher mortality [26, 31].
The influence of centre-related characteristics on patient outcomes deserves some commentary. In ICUs with more than 40 admissions/bed/year, mortality rates in patients with HA-BSI were higher. This finding may reflect delayed diagnosis, inappropriate symptomatic or aetiological treatment, unidentified factors or chance alone. Among the ICU-related factors that might influence antimicrobial use, availability of an infectious diseases specialist, antibiotic committee or written antibiotic therapy procedures did not significantly influence 28-day mortality in our study. The effectiveness of antibiotic stewardship programs in ICUs remains debated [32]. In our studies, the large proportion of ICUs having at least one physician with special training in infectious diseases may have limited our ability to detect a positive impact of antibiotic stewardship programs on patient outcomes. Studies have established that reducing antimicrobial pressure via antimicrobial stewardship programs improves the antimicrobial susceptibility of pathogens. Although such programs did not significantly influence mortality in our study, they are likely to diminish the risk for drug resistance and should be further promoted.
Several limitations of the EUROBACT study merit discussion. First, the distribution of the participating ICUs is not representative of the populations or healthcare systems in the 24 participating countries. In some countries, the number of included patients was very small. As a result, the distributions of pathogens and resistance rates reported here should be interpreted with caution. Second, each participating ICU performed laboratory tests according to their own local protocols, as opposed to sending the isolates to a central laboratory for standardised susceptibility testing. Furthermore, molecular testing of strain relatedness or confirmation of specific resistance mechanisms was not feasible in this large multinational study. Third, the study data were abstracted and entered by investigators at each ICU, with more than 100 individuals entering data in all, raising the possibility of inconsistencies. However, we attempted to minimize inconsistencies through the use of standardised definitions and of direct data entry into a web-based server. In addition, three of us (A.T., D.K., J.-F.T.) reviewed each included case for inconsistencies.
This study provides contemporary information on HA-BSI outcomes in critically ill patients within the context of increasing rates of antimicrobial resistance, particularly among gram-negative pathogens. Both MDR pathogens and failure to administer adequate antimicrobials were associated with 28-day mortality. Furthermore, the results confirm the importance of source control in severe infections. Our data underline the importance of enhanced measures to prevent HA-BSI and to control the dissemination of resistant micro-organisms. They also indicate a need for developing new antimicrobial agents for MDR gram-negative infections. The high resistance rates found in our study should encourage health authorities to preserve one of our most important resources, namely antimicrobials [33].
References
Garrouste-Orgeas M, Timsit JF, Tafflet M, Misset B, Zahar JR, Soufir L, Lazard T, Jamali S, Mourvillier B, Cohen Y, De Lassence A, Azoulay E, Cheval C, Descorps-Declere A, Adrie C, Costa de Beauregard MA, Carlet J (2006) Excess risk of death from intensive care unit-acquired nosocomial bloodstream infections: a reappraisal. Clin Infect Dis 42:1118–1126
Lambert ML, Suetens C, Savey A, Palomar M, Hiesmayr M, Morales I, Agodi A, Frank U, Mertens K, Schumacher M, Wolkewitz M (2011) Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect Dis 11:30–38
Prowle JR, Echeverri JE, Ligabo EV, Sherry N, Taori GC, Crozier TM, Hart GK, Korman TM, Mayall BC, Johnson PD, Bellomo R (2011) Acquired bloodstream infection in the intensive care unit: incidence and attributable mortality. Crit Care 15:R100
Valles J, Alvarez-Lerma F, Palomar M, Blanco A, Escoresca A, Armestar F, Sirvent JM, Balasini C, Zaragoza R, Marin M (2011) Health-care-associated bloodstream infections at admission to the ICU. Chest 139:810–815
Garrouste Orgeas M, Timsit JF, Soufir L, Tafflet M, Adrie C, Philippart F, Zahar JR, Clec’h C, Goldran-Toledano D, Jamali S, Dumenil AS, Azoulay E, Carlet J (2008) Impact of adverse events on outcomes in intensive care unit patients. Crit Care Med 36:2041–2047
Blot S, Vandewoude K, De Bacquer D, Colardyn F (2002) Nosocomial bacteremia caused by antibiotic-resistant gram-negative bacteria in critically ill patients: clinical outcome and length of hospitalization. Clin Infect Dis 34:1600–1606
Brun-Buisson C, Doyon F, Carlet J (1996) Bacteremia and severe sepsis in adults: a multicenter prospective survey in ICUs and wards of 24 hospitals. French Bacteremia-Sepsis Study Group. Am J Respir Crit Care Med 154:617–624
Laupland KB, Kirkpatrick AW, Church DL, Ross T, Gregson DB (2004) Intensive-care-unit-acquired bloodstream infections in a regional critically ill population. J Hosp Infect 58:137–145
Corona A, Bertolini G, Lipman J, Wilson AP, Singer M (2010) Antibiotic use and impact on outcome from bacteraemic critical illness: the BActeraemia Study in Intensive Care (BASIC). J Antimicrob Chemother 65:1276–1285
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 4:e296
Timsit JF, Fosse JP, Troche G, de Lassence A, Alberti C, Garrouste-Orgeat M, Bornstain C, Adrie C, Cheval C, Chevret S (2002) Calibration and discrimination of daily LOD score in predicting hospital mortality of critically ill patients, comparison with daily SOFA score. Crit Care Med 30:2003–2013
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL (2011) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. doi:101111/j.1469-0691201103570x
Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance, 2011. Available from http://www.ecdc.europa.eu/en/activities/diseaseprogrammes/ARHAI/Pages/public_consultation_clinical_microbiology_infection_article.aspx
Blakely TA, Woodward AJ (2000) Ecological effects in multi-level studies. J Epidemiol Community Health 54:367–374
Valles J, Leon C, Alvarez-Lerma F (1997) Nosocomial bacteremia in critically ill patients: a multicenter study evaluating epidemiology and prognosis. Spanish Collaborative Group for Infections in Intensive Care Units of Sociedad Espanola de Medicina Intensiva y Unidades Coronarias (SEMIUC). Clin Infect Dis 24:387–395
Souli M, Galani I, Giamarellou H (2008) Emergence of extensively drug-resistant and pandrug-resistant Gram-negative bacilli in Europe. Euro Surveill 13(47):pii:19045
Rogers BA, Aminzadeh Z, Hayashi Y, Paterson DL (2011) Country-to-country transfer of patients and the risk of multi-resistant bacterial infection. Clin Infect Dis 53:49–56
Schweizer ML, Furuno JP, Harris AD, Johnson JK, Shardell MD, McGregor JC, Thom KA, Sakoulas G, Perencevich EN (2010) Empiric antibiotic therapy for Staphylococcus aureus bacteremia may not reduce in-hospital mortality: a retrospective cohort study. PLoS ONE 5:e11432
Thom KA, Schweizer ML, Osih RB, McGregor JC, Furuno JP, Perencevich EN, Harris AD (2008) Impact of empiric antimicrobial therapy on outcomes in patients with Escherichia coli and Klebsiella pneumoniae bacteremia: a cohort study. BMC Infect Dis 8:116
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL (2008) Vincent JL (2008) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Intensive Care Med 34:17–60
Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH (2005) Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother 49:1306–1311
Vogelaers D, De Bels D, Foret F, Cran S, Gilbert E, Schoonheydt K, Blot S (2010) Patterns of antimicrobial therapy in severe nosocomial infections: empiric choices, proportion of appropriate therapy, and adaptation rates—a multicentre, observational survey in critically ill patients. Int J Antimicrob Agents 35:375–381
de Kraker ME, Davey PG, Grundmann H (2011) Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli Bacteremia: estimating the burden of antibiotic resistance in Europe. PLoS Med 8:e1001104
Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, Oh MD, Choe KW (2005) Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother 49:760–766
Zahar JR, Timsit JF, Garrouste-Orgeas M, Francais A, Vesin A, Descorps-Declere A, Dubois Y, Souweine B, Haouache H, Goldgran-Toledano D, Allaouchiche B, Azoulay E, Adrie C (2011) Outcomes in severe sepsis and patients with septic shock: pathogen species and infection sites are not associated with mortality. Crit Care Med 39:1886–1895
Linares JF, Lopez JA, Camafeita E, Albar JP, Rojo F, Martinez JL (2005) Overexpression of the multidrug efflux pumps MexCD-OprJ and MexEF-OprN is associated with a reduction of type III secretion in Pseudomonas aeruginosa. J Bacteriol 187:1384–1391
Deptula A, Gospodarek E (2010) Reduced expression of virulence factors in multidrug-resistant Pseudomonas aeruginosa strains. Arch Microbiol 192:79–84
Boyer A, Vargas F, Coste F, Saubusse E, Castaing Y, Gbikpi-Benissan G, Hilbert G, Gruson D (2009) Influence of surgical treatment timing on mortality from necrotizing soft tissue infections requiring intensive care management. Intensive Care Med 35:847–853
De Waele JJ, Hoste EA, Blot SI (2008) Blood stream infections of abdominal origin in the intensive care unit: characteristics and determinants of death. Surg Infect (Larchmt) 9:171–177
George P, Morris AM (2010) Pro/con debate: should antimicrobial stewardship programs be adopted universally in the intensive care unit? Crit Care 14:205
Carlet J, Collignon P, Goldmann D, Goossens H, Gyssens IC, Harbarth S, Jarlier V, Levy SB, N’Doye B, Pittet D, Richtmann R, Seto WH, van der Meer JW, Voss A (2011) Society’s failure to protect a precious resource: antibiotics. Lancet 378:369–371
Acknowledgments
This research project received the Clinical Research Award with a €20,000 research grant from the European Critical Care Research Network (ECCRN). The authors thank Steven Tassel for assistance in graphics design and Benjamin Keen for his open-source form management software (http://www.formtools.org/).
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript was written on behalf of the EUROBACT Study Group, whose members are listed in the Appendix.
The EUROBACT study was designed by the infection section of the European Society of Intensive Care Medicine (ESICM). The study was endorsed by the European Critical Care Research Network (ECCRN) in May 2009 and received the Clinical Research Award with a 20,000 Euro research grant from the ECCRN in November 2011.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix: members of the EUROBACT Study Group
Appendix: members of the EUROBACT Study Group
Steering committee:
Alexis Tabah, Despoina Koulenti, Stijn Blot, Jean-Ralph Zahar, Maité Garrouste-Orgeas and Jean-François Timsit
Scientific committee:
Jean Carlet, Christian Brun-Buisson, Georges Dimopoulos, Claude Martin and Jordi Rello
Statistics and methodology:
Jean Francois Timsit, Alexis Tabah and Kevin Laupland
Biostatistician: Aurélien Vesin
Study monitoring: Alexis Tabah and Sylvain Anselme
Country coordinators:
Australia: Jeffrey Lipman; Austria: Andreas Valentin; Belgium: Johan Decruyenaere; Brazil: Frederico Bruzzi de Carvalho; Canada: Kevin Laupland; China: Xiaochun Ma; Croatia: Ivan Gornik; France: Benoit Misset; Germany: Wolfgang A. Krueger; Greece: Despoina Koulenti; Hungary: Akos Csomos; Italy: Massimo Antonelli; Japan: Toshiki Mizobe; Morocco: Said Motaouakkil; The Netherlands: Marc J.M. Bonten; Poland: Adam Mikstacki; Portugal: José Artur Paiva; Republic of Serbia: Uros Batranovic; Romania: Daniela Filipescu; Spain: Jordi Valles and Ricard Ferrer; Switzerland: Philippe Eggimann; Tunisia: Fekri Abroug; Turkey: Nahit Çakar; United Arab Emirates: Hussain Al Rahma.
ICU investigators, listed by ICU (by country):
Jeffrey Lipman (PROF, MD)—Department of Intensive Care Medicine, Royal Brisbane and Women’s Hospital, Brisbane, Australia; Anne Leditschke (MD)—Helen Rodgers (RN)—Canberra Hospital Intensive Care Unit, Canberra Hospital, Canberra, Australia; David Milliss (ASC PROF)—Thomas Gottlieb (ASC PROF)—Intensive Care Services, Concord Hospital, NSW, Australia; Stuart Baker (MD)—Brigit Roberts (RN)—Icu, Sir Charles Gairdner Hospital, Perth, Australia; Peter Kraffft (MD)—Silvia Bernreiter (MD)—Intensiv 1b, Hospital Rudolfstiftung, Vienna, Austria; Pieter Depuydt (MD)—Intensieve Zorg, Universitair Ziekenhuis Gent, Ghent, Belgium; Philippe Jamaer (MD)—Icu A3 And C3, Jessa Hospital, Campus Virga Jesse, Hasselt, Belgium; Hervé Lebbinck (MD)—Iz, Az Sint Augustinus Veurne, Veurne, Belgium. Frederico Bruzzi De Carvalho (MD)—Juliana Pereira (MD)—Centro De Terapia Intensiva, Hospital Mater Dei, Belo Horizonte, Brazil; Aline Camille Yehia (MD)—Felipe Carrhá Machado (MD)—Ana Luiza Horta de sa Carneiro (MD)-Cti Hospital Julia Kubitschek, Julia Kubitschek, Belo Horizonte, Brazil; Antonio Fagundes Jr. (MD)—Unidade De Terapia Intensiva, Hospital Do Coração Do Brasil, Brasília-DF, Brazil; Fernando Rodriguez (MD)—Cti Geral, Hospital De Clínicas Niterói, Niterói, Brazil; Marcio Soares (MD, PhD)—Jorge, Salluh (MD, PhD)—Cti, Instituto Nacional De Cancer, Rio De Janeiro, Brazil. Renata Beranger (MD)—Icu, São Lucas Hospital, Rio De Janeiro, Brazil. Marcelo Lugarinho (MD)—Cti Do Hospital De Clínicas Mario Lioni, Hospital De Clínicas Mario Lioni, Rio De Janeiro, Brazil. Alexandre Carvalho (MD)—Livia, Reis (RN)—Uti 1-2-3, Udi Hospital, São Luis—Ma, Brazil. Cyntia de Lima (MD)-Uti Clínica, Hospital Santa Izabel, Salvador, Brazil. Claudio Piras (PROF, MD, PHD)—Cpc, Vitoria Apart Hospital, Vitoria, Brazil. Eliana Caser (PROF)—Jansen Falcão (MD)—Uti Geral Adulto, Centro Integrado De Atençaõ A Saúde—Cias Unimed Vitória, Vitória—ES, Brazil. Kevin B Laupland (MD)—Icu, Peter Lougheed Centre, Calgary, Canada. Kevin B. Laupland (MD)—Icu, Foothills Medical Centre, Calgary, Canada. Kevin B. Laupland (MD)—Cvicu, Foothills Medical Centre, Calgary, Canada. Kevin B. Laupland (MD)—Icu, Rockyview General Hospital, Calgary, Canada. Zhidan Zhang (MD)—Xiaochun Ma (MD)—Department of Critical Care Medicine, The First Affiliated Hospital of China Medical University, Shenyang, China. Xian Yao Wan (PROF)—Jiu Zhi Zhang (PROF)—Department of Intensive Care Medicine, The First Affiliated Hospital of Dalian Medical University, Dalian, China. Ke-Jian Qian (PROF)—Liang Xia (MD)—Intensive Care Unit, The First Affiliated Hospital of Nan Chang University, Nan Chang, China. Congshan Yang (MD)—Department of Critical Care Medicine, Zhongda Hospital, Southeast University, Nanjing, China.
Deng Lijing (MD)—Central Icu, West China Hospital of Sichuan University, Chengdu, China. Meili Duan (MD)—Department of Critical Care Medicine, Beijing Friendship Hospital, Capital Medical University, Beijing, China. Tang Zhanhong (PROF)—Pan Yiping (MD)—Intensive Care Unit, The First Hospital of Guangxi Medical University, Nanning, China. Wang Yongqiang (MD)—Luo Ning (MD)—Icu, Tianjin First Center Hospital, Tianjin, China. Zhou Li-Xin (PROF)—LI Jin-Quan (MD)—Intensive Care Unit, The Affiliated Foshan Hospital of Sun Yat-Sen University, Foshan, China. Xian Yao Wan (PROF)—Icu, Beijing Tongren Hospital, Beijing, China. Ivan Gornik (MD)—Medical Intensive Care Unit, University Hospiral Centre Zagreb, Zagreb, Croatia. Vesna Degoricija (PROF, MD, PhD)—Medical Icu, University Hospital Sisters of Mercy and University of Zagreb School of Medicine, Zagreb, Croatia.
Achille Kouatchet (MD)—Département De Réanimation Médicale Et Médecine Hy, Chu Angers, Angers Cedex 9, France. Gaetan Plantefeve (MD)—Olivier Pajot (MD)—Réanimation Polyvalente, Ch Victor Dupouy, Argenteuil, France. Hatem Kallel (MD)—Polyvalent ICU, Andrée Rosemon, Cayenne, France. Lherm Thierry (MD)—Kalfon Pierre (MD)—Réanimation Polyvalente, Louis Pasteur, Chartres, France. David Petitpas (MD)—Réanimation polyvalente, Chg Châlons En Champagne, Châlons En Champagne, France. Henry Lessire (MD)—Réanimation Médicale, Ch Pasteur, Colmar, France. Christian Brun-Buisson (PROF, MD)—Tai Pham (MD)—Réanimation Médicale, Chu Henri Mondor, Créteil, France. Djillali Annane (PROF)—Virginie Maxime (MD)—Réanimation Médicale, Chu, Garches, France. Herault Marie-Christine (MD)—Reanimation Polyvalente Chirurgicale, Chu Michallon, Grenoble, France. Sybille Merceron (MD)—Medico-Surgical ICU, André Mignot Versailles Hospital Centre, Le Chesnay, France. Eric Kipnis (ASC PROF, MD, PhD)—Marielle Boyer-Besseyre (ASC PROF, MD)—Réanimation Chirurgicale, CHRU De Lille, Lille, France. Benoit Tavernier (PROF, MD, PhD)—Sebastien Faivre (MD)—Réanimation Neurochirurgicale, CHRU de Lille, Lille, France. Voillet Francois (MD)—Renaud Lepaul Ercole (MD)—Medical Intensive Care Unit, Hopital North, Marseille, France. Vincent Willems (MD)—Réanimation Polyvalente, Centre Hospitalier De Meaux, Meaux, France. Kada Klouche (MD, PhD)—Jean Philippe Delabre (MD)—Medical Intensive Care Unit, Lapeyronie University Hospital, Montpellier, France. Cartier Julien (MD)—Gleyse Brigitte (RN)—Service De Réanimation Polyvalente, Ch De Montélimar, Montélimar, France. Sebastien Gibot (MD, PhD)—Réanimation Médicale, Hopital Central, Nancy, France. Bruno Mégarbane (PROF, MD, PhD)—Réanimation Médicale Et Toxicologique, Hôpital Lariboisière, Paris, France. Philippe Seguin (MD, PhD)—Réanimation Chirurgicale, Chu De Rennes, Rennes, France. Anne Launoy (MD)—Service De Réanimation Chirurgicale Hautepierre, Hôpitaux Universitaires De Strasbourg, Strasbourg, France. Tixier Vincent (MD)—Medical, Gabriel Montpied, Clermont-Ferrand, France. Samir Jamali (MD)—Usi (Unité De Soins Intensifs), Centre Hospitalier De Dourdan, Dourdan, France. Silvia Calvino (RN)—Alexis Tabah (MD)—Réanimation Médicale, Grenoble Teaching Hospital, Grenoble, France. Michel Durand (MD)—Marine Rossi-Blancher (MD)—Reanimation Cardiovasculaire et Thoracique, Hopital Michallon, Grenoble, France. Alexandre Debrumetz (MD)—Elie Azoulay (PROF)—Service De Réanimation Médicale, CHU Saint Louis, Paris, France. Julien Charpentier (MD)—Jean-Daniel Chiche (PROF, MD, PHD)—Réanimation Médicale Polyvalente, Cochin, Paris, France. Maité Garrouste-Orgeas (MD)—Benoit Misset (MD)—Réanimation Polyvalente, Gh Paris Saint-Joseph, Paris, France. Gernot Marx (PROF, MD)—Klinik Für Operative Intensivmedizin Und Intermediate Care, University Hospital Aachen, Aachen, Germany. Wolfgang A. Krueger (MD, PhD)—Anaesthesiology and Intensive Care Medicine, Clinics of Constance, Constance, Germany. Thomas Felbinger (MD, PhD)—Department of Anaesthesiology, The Munich Municipal Hospitals Ltd., Munich, Germany. Alexandra Heininger (MD, PhD)—ICU 20-22, Universitätsklinik Für Anaesthesiologie Und Intensivmedizin, Tuebingen, Germany. Ingo Voigt (MD)—Kardiologische Intensivstation, Elisabeth Krankenhaus Essen, Essen, Germany. Torsten Schroeder (MD)—Interdisziplinäre Intensivstation, Karl-Olga Krankenhaus, Stuttgart, Germany. Ioannis Pneumatikos (PROF, MD, PhD)—Vassiliki Theodorou (MD)—Critical Care Department, University Hospital of Alexandroupoli, Alexandroupoli, Greece. Despoina Koulenti (MD)—Apostolos Armaganidis (PROF, MD, PhD)—2nd Critical Care Department, Attikon University Hospital, Athens, Greece. Pavlos Myrianthefs (ASC PROF, MD, PhD)—Alexandra Gavala (MD)—Athens University, School of Nursing, ICU, “Kat” General Hospital, Athens, Greece. Chara Nikolaou (MD)—Katerina Kounougeri (MD)—Department of Critical Care Medicine, Konstantopouleion General Hospital of Nea Ionia, Athens, Greece. Christina Routsi (ASC PROF, MD, PhD)—Adamantia Liapikou (MD)—University ICU Department, Evangelismos General Hospital, Athens, Greece. Christodoulos Nathanail (MD, PhD)—Pirros Tsakas (MD)—Intensive Care Unit, General Hospital of Arta, Arta, Greece. Andreas Karabinis (MD, PhD)—Christos Tsakalakis (MD)—Critical Care Department, General Hospital “G. Genimatas,” Athens, Greece. Kostas Mandragos (MD, PhD)—Chrysostomos Katsenos (MD)—Intensive Care Unit, Red Cross (Erythros Stavros) Hospital, Athens, Greece. Georgios Anthopoulos (MD)—Georgios Choutas (MD)—Intensive Care Unit, 251 Air Force General Hospital, Athens, Greece. Anastasia Koutsikou (MD)—Ilona Nikolaidou (MD)—Intensive Care Unit (Surgical), General Hospital of Athens “Asklepieion Voulas,” Athens, Greece. Vasileios Bekos (MD)—Anna Spring (RN)—Intensive Care Unit, Naval Hospital of Athens, Athens, Greece. Haralambos Paskalis (MD)—Vassiliki Psallida (MD)—Intensive Care Unit, Hygeia General Hospital, Athens, Greece. Aikaterini Ioakeimidou (MD)—Alexandra Lahana (MD, PhD)—Intensive Care Unit, Athens Veterans Hospital (Nimits), Athens, Greece. Paraskevi Plantza (MD)—Aikaterini Nodarou (MD)—Icu Kaa Sotiria General Hospital, Sotiria General Hospital, Athens, Greece. Antonia Koutsoukou (ASC PROF, MD, PhD)—Magdalini Kyriakopoulou (MD)—ICU 1st Resp. Medicine Depart. Athens University, Sotiria General Hospital, Athens, Greece. Martha Michalia (MD)—Phyllis Clouva-Molyvdas (MD)—Department of Critical Care Medicine, “Thriassion” General Hospital of Eleusis, Elefsina, Athens, Greece. Dimitrios Sfyras (MD)—Christos Georgiadis (MD)—Intensive Care Unit, General Hospital of Lamia, Lamia, Greece. Pavlos Polakis (MD)—Spiros Papanikolaou (MD)—Intensive Care Unit, “Peiraiko” Therapeftirio, Pireus, Greece. Christos Christopoulos (MD, PhD)—Efstratia Vrettou (MD)—Intensive Care Unit, General Hospital of Pyrgos, Pyrgos, Greece. Kostoula Arvaniti (MD, PhD)—Dimitrios Matamis (MD, PhD)—Critical Care Department, “Papageorgiou” General Hospital of Thessaloniki, Thessaloniki, Greece. Theoniki Paraforou (MD, PhD)—Kyriaki Spiropoulou (MD)—ICU, General Hospital of Trikala, Trikala, Greece. Dimitris Georgopoulos (PROF, MD, PhD)—Maria Klimathianaki (MD)—Critical Care Department, University General Hospital of Heraklion, Crete, Heraklion, Greece. Georgios Nakos (PROF, MD, PhD)—Vasilios Koulouras (ASC PROF, MD, PhD)—Critical Care Department, University Hospital of Ioannina, Ioannina, Greece. Apostolos Komnos (MD, PhD)—Achilleas Chovas (MD, PhD)—Department of Critical Care Medicine, General Hospital of Larisa, Larisa, Greece. Athanasios Prekates (MD, PhD)—Eleni Magira (MD, PhD)—Critical Care Department, “Tzaneion” General Hospital of Pireus, Pireus, Greece. Maria Giannakoy (MD, PhD)—Eleni Gkeka (MD)—Intensive Care Unit, “Ahepa” General Hospital, Thessaloniki, Greece. Eleni Antoniadou (MD, PhD)—Elli Antypa (MD, PhD)—Critical Care Department, General Hospital of Thessaloniki “G. Genimatas,” Thessaloniki, Greece. Nikoletta Gritsi-Gerogianni (MD, PhD)—Christina Kydona (MD)—Critical Care Department, “Hippokrateion” General Hospital of Thessaloniki, Thessaloniki, Greece. Epaminondas Zakynthinos (ASC PROF, MD, PhD)—Nikolas Tzovaras (MD)—Critical Care Department, University Hospital of Larissa, Larissa, Greece. Akos Csomos (MD, PhD)—Surgical Intensive Care, Semmelweis University, Budapest, Hungary. Csóka Gábor (MD)—Sürgősségi Beteg Ellátó Egység, Fővárosi Önkormányzat Szent Imre Kórház, Budapest, Hungary. Borbala Mikos (MD)—György Velkey (MD)—Pediatric Anaesthesia And Intensive Care Unit, Bethesda Children’s Hospital of The Hungarian Reformed Church, Budapest, Hungary. Eszter Vitális (MD)—Auguszta Sebészet Intenzív, The Medical And Health Science Centre of the University of Debrecen, Debrecen, Hungary. Nóra, Ágota Kovács (MD)—Aito Flór Ferenc, Kistarcsa, Hungary. Lajos Bogar (PROF, MD, PHD)—Tamas Kiss (MD)—Department of Anaesthesia and Intensive Therapy, University of Pécs 400 Bed Clinic, Pécs, Hungary. Zollei Eva (MD)—Medical ICU, University of Szeged, Szeged, Hungary. Valerio Mangani (MD)—Giorgio Tulli (Prof)—Intensive Care Unit, S.Giovanni Di Dio, Firenze, Italy. Guido Stefania (MD)—Ronco Chiara (STUDENT)—Centro Rianimazione, Maggiore Della Carità, Novara, Italy. Massimo Antonelli (PROF)—Luca Montini (MD)—Rianimazione E Terapia Intensiva, Policlinico Universitario A. Gemelli, Rome, Italy. Monica Rocco (PROF, MD)—Giorgia Citterio (MD)—Centro Di Rianimazione, Policlinico Umberto I, Rome, Italy. Shigeki Fujitani (MD)—Emergency & Critical Care Medicine, St.Marianna University School of Medicine Hospital, Kanagawa, Japan. Koji Hosokawa (MD)—Intensive Care Unit, Kyoto Prefectural University of Medicine, Kyoto, Japan. Motaouakkil Said (PROF)—Charra Boubaker (PROF)—Reanimation Medicale, CHU Ibn Rochd, Casablanca, Morocco. Marcus Schultz (PROF, MD, PhD, FCCP)—Annelou Van Der Veen (RN)—icu, Academic Medical Center, Amsterdam, The Netherlands. Heleen Aardema (MD)—Intensive and Respiratory Care Unit, University Medical Center Groningen, Groningen, The Netherlands. Dennis Bergmans (MD, PhD)—Rik Schoemakers (BSC)—Department of Intensive Care, Maastricht University Medical Centre, Maastricht, The Netherlands. Ronald Wesselink (MD, PhD)—Icu, St. Antonius Ziekenhuis, Nieuwegein, The Netherlands. Evelien A.N. Oostdijk (MD)—Marc J.M., Bonten (PROF, MD, PHD)—Department of Medical Microbiology, University Medical Center Utrecht, Utrecht, The Netherlands. Iwona Dragan (MD)—Icu, General Hospital, Gniezno, Poland. Włodzimierz Kostyrka (MD)—Micu, Szpital Powiatowy, Ostrów Wielkopolski, Poland. Barbara Tamowicz (MD, PhD)—Adam Mikstacki (MD, PhD)—Department of Anaesthesiology and Intensive Therapy, Poznan University of Medical Sciences, Regional Hospital, Poznan, Poland. Piotr Smuszkiewicz (MD, PhD)—Department of Anaesthesiology and Intensive Therapy, University Hospital, Poznan, Poland. Jacek Nadolski (MD, PhD)—Oa I It, Wielkopolska Center of Pulmonology and Thoracic surgery, Poznań, Poland. Robert Choma (MD)—Oddział Anestezjologii I Intensywnej Terapii, Szpital W Śremie, Śrem, Poland. Wladyslaw Koscielniak (MD)—Pawel Pietraszek (MD)—Oddzial Anestezjologii I Intesywnej Terapii, Regional Hospital Zielona Gora, Zielona Gora, Poland. Edward Maul (MD)—Serviço De Medicina Intensiva, Hospital Central Do Funchal, Funchal, Portugal. Anabela Bártolo (MD)—Salomé Martins—Ucip, Chaa—Guimarães, Guimarães, Portugal. Isabel Miranda (MD)—Mariana Oliveira ()—Ucip02, Hospital De St. António Dos Capuchos, Centro Hospitalar De Lisboa Central, E.P.E., Lisboa, Portugal. Carlos França (PROF)—Ana Tornada (MD)—Smi, Hospital De Santa Maria, Lisbon, Portugal. Luís Telo (MD)—Leonardo Ferreira (MD)—Ucip, Pulido Valente, Lisboa, Portugal. Teresa Cardoso (MD)—Unidade De Cuidados Intensivos Polivalente, Hospital De Santo António, Porto, Portugal. Lurdes Santos (PhD)—Alcina Ferreira (MD)—Uci-Di, Hospital S. João, Porto, Portugal;;José Manuel Pereira (MD)—Ucip Geral, Hospital S João, Porto, Portugal; Celeste Dias (MD)—Uci Neurocriticos, Hospital Sao Joao, Porto, Portugal. Maria Conceição Dias (MD)—Ucipu—Ucip Urgencia, Hospital De S. João, Porto, Portugal. Ana J. Marques (MD)—Paula Castelões (MD)—Ucipolivalente Do Chvngaia, Hospital Santos Silva—Centro Hospitalar Vila Nova De Gaia, Vila Nova Gaia, Portugal. Uros Batranovic (MD)—Srdjan Gavrilovic (MD)—Intensive Care Unit, Institute For Pulmonary Diseases of Vojvodina, Sremska Kamenica, Republic of Serbia. Daniela Filipescu (PROF, MD, PhD)—Cardiac Anesthesia And Intensive Care, Emergency Institute of Cardiovascular Diseses, Bucharest, Romania. Francisco Alvarez-Lerma (PhD)—Maria Pilar Gracia (PhD)—Intensive Care Unit, Hospital Del Mar, Barcelona, Spain. Fernando Armestar-Rodriguez (MD)—Eduard, Mesalles-Sanjuán (MD)—Medicina Intensiva, Hospital Universitari Germans Trias I Pujol, Badalona, Spain;Nerea Lopez De Arbina (MD)—Josep Sirvent (MD)—Servicio De Medicina Intensiva, Hospital Universitari De Girona Dr Josep Trueta, Girona, Spain. Pau Garro (MD)—Uci General, Hospital General De Granollers, Granollers (Barcelona), Spain. Juan Ramón Cortés Cañones (MD)—Unidad Cuidados Intensivos, Complexo Hospitalario Ourense Cristal Piñor, Orense, Spain. Armando Blanco (MD, PhD)—Lara Marqués (MD, PhD)—Unidad De Medicna Intensiva I, Hospital Universitario Central De Asturias (Huca), Oviedo, Spain. Josu Insausti (MD)—Iñigo Martija (MD)—Uci, Hospital De Navarra, Pamplona, Spain. Jordi Valles (MD, PhD)—Ricard Ferrer (MD, PhD)—Critical Care Center, Hospital Sabadell, Sabadell, Spain. Alejandro Ubeda (MD)—Francisco Lucena (MD)—Polyvalent ICU, H.U. Valme, Seville, Spain. Maricarmen Gilavert Cuevas (MD)—Uci, Hospital Universitario Joan Xxiii-Instituto Pere Virgili, Tarragona, Spain. Rafael Zaragoza (MD)—Susana Sancho (MD) – ICU, Hospital Univ. Dr. Peset, Hospital Universitario Dr. Peset, Valencia, Spain. Markus Laube (MD)—Madeleine Rothen (MD)—Intensivstation, Spitalzentrum, Biel, Switzerland. Philippe Eggimann (MD)—Jean-Luc Pagani (MD)—Service De Médecine Intensive Adulte, Chuv, Lausanne, Switzerland. Samia Ayed (MD)—Service De Reanimation Medicale, CHU Tahar Sfar, Mahdia, Tunisia. Islem Ouanes (MD)—Fekri Abroug (PROF)—Réanimation Polyvalente, Chu Fattouma Bourguiba, Monastir, Tunisia. Dilek Özcengiz (PROF)—Reanimation, Cukurova Medical University, Adana, Turkey. Seyhan Yağar (MD, PhD)—Cardiovascular Surgery, ICU, Türkiye Yüksek Ihtisas Hospital, Ankara, Turkey. Süheyla Ünver (MD, ASC PROF)—Yeliz Irem Tunçel (MD)—Anestesia Intensive Care Unit, Ankara Dr Abdurrahman Yurtaslan Onkoloji E. A. Hastanesi, Ankara, Turkey. Unase Buyukkocak (PROF, MD)—Esra Aykac (RESIDENT)—Intensive Care Unit and Anaesthesia, Kirikkale University, The School of Medicine Hospital, Kirikkale, Turkey. Ahmet COŞAR (PROF, MD)—Hüseyin Oğuz Yilmaz (MD)—Anesteziyoloji Ve Reanimasyon Ad Ybü, Gülhane Askeri Tıp Akademisi, Ankara, Turkey. Arash Pirat (ASC PROF)—Pinar Zeyneloglu (ASC PROF)—Surgical Intensive Care Unit, Baskent University Hospital, Ankara, Turkey. Nermin Kelebek Girgin (MD)—Halis Akalın (PROF, MD)—Anaesthesiology and ICU, Uludag University Medical Faculty, Bursa, Turkey. Hulya Sungurtekin (MD, PROF)—Simay Serin (PROF, MD)—Anaesthesiology and ICU, Pamukkale University, Denizli, Turkey. I. Ozkan Akinci (ASC PROF)—Neuro Icu, Istanbul Medical Faculty, Istanbul, Turkey. Tayfun ADANIR (MD)—Atilla Sencan (MD)—Anaesthesiology and ICU, Ataturk Training And Research Hospital, Izmir, Turkey. Ahmet Dilek (ASC PROF, MD)—Mikail Yüksel Intensive Care Unit, Ondokuz Mayis University, School of Medicine, Samsun, Turkey. Ismail KATI (ASC PROF)—Ugur Goktas (ASC PROF)——Anaesthesia and Intensive Care Unit, Yuzuncu Yil University Medical Faculty, Van, Turkey. Ashraf El Houfi (MD, MS, FRCP)—Micu-Sicu, Dubai Hospital, Dubai, United Arab Emirates.
Rights and permissions
About this article
Cite this article
Tabah, A., Koulenti, D., Laupland, K. et al. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med 38, 1930–1945 (2012). https://doi.org/10.1007/s00134-012-2695-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2695-9