Abstract
Purpose
To assess the associations between a priori-selected markers of inflammation and coagulation and delirium during critical illness.
Methods
In this prospective cohort study, we collected blood from mechanically ventilated medical intensive care unit (ICU) patients and measured nine plasma markers of inflammation and coagulation. We assessed patients daily for delirium using the Confusion Assessment Method for the ICU and used multivariable regression to analyze the associations between plasma markers and subsequent delirium, after adjusting for age, severity of illness, and sepsis.
Results
Among the 138 patients studied, with median age of 66 years and median Acute Physiology and Chronic Health Evaluation (APACHE) II of 27, 107 (78 %) were delirious at some point during the study. Two markers of inflammation and one of coagulation were significantly associated with delirium. After adjusting for covariates, lower plasma concentrations of matrix metalloproteinase-9 (MMP-9) and protein C were associated with increased probability of delirium (p = 0.04 and 0.01, respectively), and higher concentrations of soluble tumor necrosis factor receptor-1 (sTNFR1) were associated with increased probability of delirium (p < 0.01). Concentrations of C-reactive protein (p = 0.82), myeloperoxidase (p = 0.11), neutrophil gelatinase-associated lipocalin (p = 0.70), D-dimer (p = 0.83), plasminogen activator inhibitor type 1 (p = 0.98), and Von Willebrand factor antigen (p = 0.65) were not associated with delirium.
Conclusions
In this study, MMP-9, protein C, and sTNFR1 were independently associated with subsequent ICU delirium. These results suggest that specific aspects of inflammation and coagulation may play a role in the evolution of delirium during critical illness and that these markers should be examined in larger studies of ICU patients.
Similar content being viewed by others
Introduction
Delirium, an acute form of brain dysfunction or cerebral insufficiency [1] affecting 60–80 % of mechanically ventilated patients in the intensive care unit (ICU), is both pervasive and foreboding for the large and rapidly growing population of critically ill patients worldwide [2]. In addition to promoting adverse events, such as self-extubation [3], delirium delays extubation [4] and discharge from the ICU [4] and hospital [5] and is independently associated with increases in mortality [6–8] and cognitive impairment months to years after critical illness [9]. Despite the impact of delirium on outcomes and costs [10]—and despite the development of reliable and valid tools to identify delirium [11, 12]—little is known regarding the biological mechanisms that lead to this form of organ dysfunction during critical illness.
Studies among older non-ICU patients suggest that inflammation plays a role in the pathophysiology of delirium [13–15]. Though its acute effects on the brain are poorly understood, inflammation is known to cause dysfunction of other organs during critical illness [16], in part by stimulating a process of deranged coagulation, whose end product is microvascular damage and thrombosis in vital organs, such as the kidneys [17] and lungs [18]. The blood–brain barrier and brain are likely affected as well. Animal models show that inflammatory mediators circulating systemically during sepsis readily cross the blood–brain barrier [19]. It is suspected, therefore, that the systemic inflammation and attendant coagulation that commonly characterize critical illness are important contributors to the development and continuation of delirium [20–22].
To explore the hypothesis that inflammation and deranged coagulation are risk factors for delirium during critical illness, we assessed the associations between five markers of inflammation and four markers of coagulation and delirium during critical illness in a prospective cohort study, seeking to generate hypotheses about specific biomarkers for definitive testing in larger studies. In light of our overarching hypothesis, we expected that delirium would be predicted by elevations in each of the plasma markers studied, except for protein C, which we expected to be reduced in association with delirium [23].
Patients and methods
Study design and population
We conducted this nested prospective cohort study at a single center (Saint Thomas Hospital, Nashville, TN, USA) participating in the Awakening and Breathing Controlled trial (ClinicalTrials.gov number NCT00097630) [24], a randomized trial assessing the efficacy of a paired sedation and ventilator weaning protocol. All medical ICU patients mechanically ventilated >12 h were eligible for enrollment except patients admitted after cardiopulmonary arrest, moribund patients, those with profound neurological deficits (e.g., due to large stroke or severe dementia), patients enrolled in another clinical trial, and those whose current episode of mechanical ventilation had lasted ≥2 weeks. At the time of enrollment, we obtained informed consent from authorized surrogates because most participants did not have capacity to consent; when capable, patients could consent or withdraw. The institutional review boards at Saint Thomas Hospital and Vanderbilt University Medical Center (Nashville, TN, USA), site of the coordinating center, approved the study protocol.
Measurement of inflammation and coagulation
On the morning after enrollment (or, in the rare case that blood could not be collected at that time, within 2 days after enrollment), we collected initial venous blood samples from patients using ethylenediaminetetraacetic acid (EDTA) tubes for inflammatory markers and citrate tubes for markers of coagulation. Within 4 h of collection, the tubes were centrifuged; plasma was removed and stored at −80 °C until batched measurement of biomarkers. We collected a second follow-up blood sample on study day 5, except when unavailable due to discharge or death.
A priori, we selected five markers of inflammation and four markers of coagulation—all nine markers are described in the Electronic Supplementary Material (ESM)—based on previous studies examining inflammation and coagulation during critical illness. Markers of inflammation included (1) C-reactive protein (CRP), (2) matrix metalloproteinase-9 (MMP-9), (3) myeloperoxidase (MPO), (4) neutrophil gelatinase-associated lipocalin (NGAL), and (5) soluble tumor necrosis factor receptor-1 (sTNFR1). Markers of deranged coagulation included (1) D-dimer, (2) protein C, (3) plasminogen activator inhibitor type 1 (PAI-1), and (4) Von Willebrand factor antigen (VWF).
A detailed description of the methods used to measure biomarkers is provided in the ESM. All laboratory personnel were blinded to patients’ clinical characteristics and outcomes, including delirium.
Measurement of covariates and outcome
To adjust for potential confounders, we selected covariates a priori based on biological plausibility and previous research [25]. These covariates, collected at enrollment, included age, severity of illness, and admission with severe sepsis, which was identified according to treating physicians’ diagnosis and confirmed using consensus criteria [26]. Severity of illness was measured using the acute physiology score (APS) of the Acute Physiology and Chronic Health Evaluation (APACHE) II score [27].
Using the Confusion Assessment Method for the ICU (CAM-ICU) [28, 29], research personnel assessed patients for delirium daily in the morning from study day 1 until death or ICU discharge. Based on the CAM-ICU, delirium was categorized as present or absent on each study day that the patient was not comatose according to the Richmond Agitation–Sedation Scale (RASS) [30, 31]; RASS −4 or −5 were considered coma. When a neurologic assessment was missing—which was the case for only 1.9 % of all patient-days—mental status was assigned for that day using multiple imputation [32] that relied on mental status observed the day prior to the missing assessment and patient status the day after the missing assessment, e.g., observed mental status, ICU discharge, or death. None of the biomarker results were known at the time patients were assessed for delirium.
Statistical analysis
Our primary goal in this hypothesis-generating study was to narrow the field of inflammatory and coagulation markers that should be studied in large, multicenter investigations. To determine which candidate markers of inflammation and coagulation (the continuous exposure variables of interest) are independently associated with delirium (the dichotomous outcome), we used logistic regression with generalized estimating equations (GEE) to analyze the probability of being delirious the day following each biomarker measurement, adjusting for age, severity of illness, and severe sepsis. Since both exposures and outcomes were measured more than once per patient, GEE was used to account for correlation between multiple observations from the same patient. We specifically analyzed the temporal (i.e., before–after) associations between biomarkers and delirium assessed within 24 h after biomarker measurement; if a patient could not be assessed for delirium on a particular day (e.g., because of coma, discharge, or death), the biomarkers measured on the previous day were excluded from analysis.
To reduce the risk of type I error, we performed a global test based on Wald statistics, which assesses the combined association of the nine biomarkers with delirium after adjusting for covariates. We then included each plasma marker individually in a separate logistic regression model with GEE to avoid multicollinearity, assessing for associations between individual biomarkers and delirium.
In addition to the primary analyses, we performed several sensitivity analyses, which are described, along with other details regarding the statistical analyses, in the ESM. We used R (version 2.11.1) for all statistical analyses [33].
Results
From March 2004 to March 2006, blood was collected from 138 mechanically ventilated medical ICU patients whose baseline characteristics are presented in Table 1. The majority of patients were 65 years old or older, and one-quarter were ≥75 years old. Nearly half were admitted with severe sepsis and/or acute respiratory distress syndrome. Delirium was common, with 78 % of patients being delirious at some point during their ICU stay. On the days of biomarker measurement included in the analyses, most patients were alert or mildly sedated; the median [interquartile range] RASS on these days was 0 [−2 to 0].
Initial and follow-up plasma marker concentrations are displayed in Table 2. Nineteen (14 %) patients died within 5 days of enrollment such that only the initial biomarker concentration could be obtained for these patients.
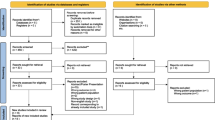
After adjusting for age, severity of illness, and severe sepsis, the Wald global test found that the group of nine plasma markers studied was significantly associated with delirium (p = 0.02), indicating that one or more markers was associated with delirium and allowing us to proceed with examining the associations of individual biomarkers with delirium. In separate models including individual biomarkers, two inflammatory markers and one marker of coagulation were significantly associated with delirium (Table 3). Higher MMP-9 concentrations were associated with reduced probability of delirium (p = 0.04). Figure 1a shows the association, which was nonlinear; an increase in MMP-9 from 0.20 to 20 ng/mL (for this nonlinear association, values for comparison were selected based on the graph) was not significantly associated with a change in probability of delirium [odds ratio (OR) 1.3, 95 % confidence interval (CI) 0.2–9.3], whereas an increase from 20 to 300 ng/mL was associated with a significant decrease in probability of delirium [OR 0.3, 95 % CI 0.1–0.7]. As shown in Fig. 1b, higher concentrations of sTNFR1 were associated with increased probability of delirium (p < 0.01); an increase in sTNFR1 from 2,900 to 6,302 pg/mL (values for comparison are the 25th and 75th percentiles) was associated with a 2.1-fold increase in the odds of delirium (95 % CI 1.2–3.6). Finally, low protein C was associated with increased probability of delirium (p = 0.01). Figure 1c shows this nonlinear association; an increase in protein C from 60 to 140 % of the pooled control plasma standard was associated with a 90 % decrease in the odds of delirium (OR 0.1, 95 % CI 0–0.4), whereas an increase from 140 to 200 % control was not significantly associated with a change in the odds of delirium (OR 3.3, 95 % CI 0.9–12.2).
Plasma markers associated with delirium. The probability of delirium that was independently associated with matrix metalloproteinase-9 (MMP-9), soluble tumor necrosis factor receptor-1 (sTNFR1), and protein C concentrations, after adjusting for age, severity of illness, and sepsis at admission is indicated by solid black lines. The gray ribbons indicate the 95 % confidence limits of these associations. Each black circle represents an observation with position along the x axis indicating the biomarker value and position along the y axis indicating presence (y = 1) or absence (y = 0) of delirium. Panel a shows that MMP-9 had a significant nonlinear association with delirium (p = 0.04). Panel b shows that sTNFR1, which was log transformed to improve model fit, had a significant linear association with delirium (p < 0.01). Panel c shows that protein C had a significant nonlinear association with delirium (p = 0.01).
The sensitivity analyses found similar results to the primary analyses; detailed results are provided in the ESM.
Discussion
In this prospective cohort study, MMP-9, sTNFR1, and protein C were independently associated with delirium during critical illness. Though the specific biological significance of these associations is as yet unknown, these findings lend support to the hypothesis that inflammation and deranged coagulation are related to delirium and suggest that certain plasma markers might be useful in the study of the pathogenesis of delirium, as diagnostic or prognostic tools, or as potential targets for therapeutic interventions.
Delirium was predicted by elevated sTNFR1. Numerous experimental models show that TNF-alpha is an important mediator of organ dysfunction during critical illness, but this proinflammatory cytokine has not been consistently associated with outcomes in human studies [34, 35], perhaps due to its rapid decline to undetectable levels after an initial increase early during illness. We chose therefore to study sTNFR1, which is released into circulation from cells after activation by TNF-alpha, because it is measurable for days in most critically ill patients and serves as a marker of the proinflammatory state. Though sTNFR1 had not been previously studied as a marker of delirium, our results are consistent with studies that have found other plasma markers of inflammation to be associated with delirium in older hospitalized non-ICU patients [13, 14], postoperative patients [15, 36], and critically ill patients [37–40]. Notably, only one other ICU study [40] adjusted for severity of illness, a potential confounder associated with both inflammation and delirium. Our analyses found sTNFR1 to be an important independent predictor of delirium even after accounting for age, sepsis, and severity of illness. Larger studies are needed to confirm our findings and determine which marker of inflammation is most useful for prediction and/or diagnosis of delirium, since the current results suggest that not all markers are consistent predictors.
Though some studies of non-ICU patients [14, 15] found that CRP was associated with delirium, ICU studies have yielded conflicting results. Our study found that CRP was not associated with delirium in the ICU after adjusting for potential confounders, including severity of illness, a result consistent with two recent ICU studies finding no association [40, 41]. Alternatively, two other studies found CRP was higher in delirious ICU patients than in those without delirium, but neither adjusted for severity of illness so the reported associations may be due to confounding [37, 38]. In sum, this evidence might indicate that nonspecific acute-phase reactants such as CRP are not consistently useful in the study of delirium in the ICU. It is also possible that systemic inflammation does not play a role in delirium, which would mean that complex mechanisms other than inflammation are responsible for the association between sTNFR1 and delirium. In addition to its role in immune signaling, TNF-alpha interacts with receptors and ion channels in the brain to regulate neuronal excitability and synaptic plasticity [42]. Thus, disease-induced changes in levels of TNF-alpha in the circulation and the central nervous system may contribute to delirium via disruption of the cytokine’s normal adaptive roles in the brain.
Our finding that low protein C was associated with increased probability of delirium might reflect a role of deranged coagulation in the pathogenesis of delirium during critical illness, since activated protein C blocks microvascular coagulation and low protein C concentrations therefore are associated with increased coagulation [23]. Alternatively, our finding could result from one or more of protein C’s pleiotropic effects. In addition to numerous anti-inflammatory properties—including suppression of proinflammatory cytokines and inhibition of leukocyte adhesion and chemotaxis [43]—activated protein C has been found to have neuroprotective effects, which are attributed to attenuation of glutamate-induced excitotoxicity [44] and prevention of neuronal apoptosis [45]. Thus, the association between low protein C and delirium shown in our study could reflect a role of increased coagulation, increased inflammation, and/or reduced neuroprotection in delirium during critical illness. Any of these effects may explain the results of a recent observational study that suggested treatment with recombinant human activated protein C [drotrecogin alfa (activated)—a drug not available for clinical use] may reduce sepsis-associated delirium or encephalopathy [46].
Unlike the findings regarding sTNFR1 and protein C, the association between lower concentrations of MMP-9 and delirium was unexpected; elevated MMP-9 is generally considered an indicator of inflammation, so we hypothesized that elevated MMP-9 would be associated with delirium. One possible explanation for our finding is that the relationship between MMP-9 and delirium during critical illness may be influenced more by MMP-9’s direct role in brain function than by its role in inflammation. Like leukocytes, neurons can express this protease, which operates extracellularly in the brain after being released in response to enhanced neuronal activity [47]. Animal models suggest that MMP-9 plays an important physiologic role in neuroplasticity [48, 49], making it important in memory and learning, processes that are frequently impaired during delirium. It remains unclear, however, whether the normal physiologic effects of MMP-9 remain intact during critical illness or if the enzyme promotes damage in this setting. Though our results are more consistent with the former, studies of MMP-9 in the setting of other neurologic diseases or conditions have found the marker to be elevated, e.g., in Alzheimer’s disease [50], stroke [51], and traumatic brain injury [52]. Our finding may also result from confounding due to unmeasured covariates. Statins, for example, have been reported to reduce MMP-9 activity [53] and may have effects on delirium during critical illness [54, 55]. Future studies are needed to clarify the role of MMP-9 in brain function during critical illness after accounting for potential confounders, including statin exposure.
Strengths of this investigation include measurement of plasma markers before assessment of outcomes; use of a well-validated tool to diagnose delirium; and a diverse population of medical ICU patients, with varying ages and diagnoses. One limitation was our measurement of biomarkers twice rather than daily, preventing detailed examinations of kinetic profiles and testing of additional hypotheses, such as the hypothesis recently proposed on the basis of a study involving healthy volunteers [56], i.e., that sustained elevation of proinflammatory cytokines is more important to the pathophysiology of brain dysfunction during critical illness than the absolute peak reached by these cytokines. Also, though we studied nine separate markers, limitations in funding prevented us from measuring other inflammatory markers of interest; interleukin (IL)-6 [39] and IL-8 [57], for example, warrant examination in future studies of delirium during critical illness. Other limitations include measurement of circulating levels of markers in plasma rather than levels in the cerebrospinal fluid, which would presumably be more reflective of changes in the brain but which would be difficult to obtain [58]; potential confounding by hemodialysis (which was not tracked in the study and which may alter some biomarker concentrations [59]) or by death or coma, outcomes that may be increased by inflammation and which prevent assessment for delirium; a single-center study design, which may limit generalizability; and our separate analyses of multiple biomarkers. In a single model that is less subject to type I error than multiple separate models, the Wald global test was significant, indicating that one or more plasma markers was associated with delirium. However, we also examined nine markers in separate models, which could result in false positives. We chose not to adjust for these multiple comparisons, in keeping with authoritative recommendations [60] and to avoid type II errors, since we planned for our results to direct future research rather than directly inform clinical practice.
In conclusion, this investigation found that three plasma markers of inflammation and coagulation, MMP-9, sTNFR1, and protein C, are associated with delirium in the ICU. These results, which lend support to the hypothesis that inflammation and deranged coagulation are important mechanisms of delirium during critical illness, need confirmation and further study in larger, multicenter investigations of critically ill patients with and without severe sepsis. Additionally, related markers should be studied to enhance understanding of the mechanisms of delirium, and potential associations between inflammatory markers and other manifestations of brain dysfunction, including coma, should be examined, since delirium is one of several manifestations of acute brain dysfunction that occur during critical illness [61].
References
Engel GL, Romano J (1959) Delirium, a syndrome of cerebral insufficiency. J Chronic Dis 9:260–277
Girard TD, Pandharipande PP, Ely EW (2008) Delirium in the intensive care unit. Crit Care 12(Suppl 3):S3
Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y (2001) Delirium in an intensive care unit: a study of risk factors. Intensive Care Med 27:1297–1304
Shehabi Y, Riker RR, Bokesch PM, Wisemandle W, Shintani A, Ely EW (2010) Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med 38:2311–2318
Ely EW, Gautam S, Margolin R, Francis J, May L, Speroff T, Truman B, Dittus R, Bernard R, Inouye SK (2001) The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med 27:1892–1900
Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr, Inouye SK, Bernard GR, Dittus RS (2004) Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 291:1753–1762
Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y (2007) Incidence, risk factors and consequences of ICU delirium. Intensive Care Med 33:66–73
Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH (2009) Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med 180:1092–1097
Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, Gordon SM, Canonico AE, Dittus RS, Bernard GR, Ely EW (2010) Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med 38:1513–1520
Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, Truman B, Bernard GR, Dittus RS, Ely EW (2004) Costs associated with delirium in mechanically ventilated patients. Crit Care Med 32:955–962
van Eijk MM, van Marum RJ, Klijn IA, de Wit N, Kesecioglu J, Slooter AJ (2009) Comparison of delirium assessment tools in a mixed intensive care unit. Crit Care Med 37:1881–1885
Luetz A, Heymann A, Radtke FM, Chenitir C, Neuhaus U, Nachtigall I, von Dossow V, Marz S, Eggers V, Heinz A, Wernecke KD, Spies CD (2010) Different assessment tools for intensive care unit delirium: which score to use? Crit Care Med 38:409–418
de Rooij SE, van Munster BC, Korevaar JC, Levi M (2007) Cytokines and acute phase response in delirium. J Psychosom Res 62:521–525
Macdonald A, Adamis D, Treloar A, Martin F (2007) C-reactive protein levels predict the incidence of delirium and recovery from it. Age Ageing 36:222–225
Beloosesky Y, Hendel D, Weiss A, Hershkovitz A, Grinblat J, Pirotsky A, Barak V (2007) Cytokines and C-reactive protein production in hip-fracture-operated elderly patients. J Gerontol A Biol Sci Med Sci 62:420–426
Marshall JC (2001) Inflammation, coagulopathy, and the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med 29:S99–S106
Schrier RW, Wang W (2004) Acute renal failure and sepsis. N Engl J Med 351:159–169
Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. N Engl J Med 342:1334–1349
Papadopoulos MC, Lamb FJ, Moss RF, Davies DC, Tighe D, Bennett ED (1999) Faecal peritonitis causes oedema and neuronal injury in pig cerebral cortex. Clin Sci (Lond) 96:461–466
Maclullich AM, Ferguson KJ, Miller T, de Rooij SE, Cunningham C (2008) Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res 65:229–238
Cerejeira J, Firmino H, Vaz-Serra A, Mukaetova-Ladinska EB (2010) The neuroinflammatory hypothesis of delirium. Acta Neuropathol 119:737–754
van Gool WA, van de Beek D, Eikelenboom P (2010) Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet 375:773–775
Esmon CT (2001) Protein C anticoagulant pathway and its role in controlling microvascular thrombosis and inflammation. Crit Care Med 29:S48–S52
Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, Taichman DB, Dunn JG, Pohlman AS, Kinniry PA, Jackson JC, Canonico AE, Light RW, Shintani AK, Thompson JL, Gordon SM, Hall JB, Dittus RS, Bernard GR, Ely EW (2008) Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 371:126–134
Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, Bernard GR, Ely EW (2006) Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology 104:21–26
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med 31:1250–1256
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, Hart RP, Dittus R (2001) Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the intensive care unit (CAM-ICU). JAMA 286:2703–2710
Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, Inouye SK (2001) Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the intensive care unit (CAM-ICU). Crit Care Med 29:1370–1379
Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK (2002) The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 166:1338–1344
Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, Francis J, Speroff T, Gautam S, Margolin R, Sessler CN, Dittus RS, Bernard GR (2003) Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation–Sedation Scale (RASS). JAMA 289:2983–2991
Harrell FE Jr (2001) Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. Springer, New York
Ihaka R, Gentlemen R (1996) R: a language for data analysis and graphics. J Comput Graph Stat 5:299–314
Parsons PE, Matthay MA, Ware LB, Eisner MD (2005) Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol 288:L426–L431
De Freitas I, Fernandez-Somoza M, Essenfeld-Sekler E, Cardier JE (2004) Serum levels of the apoptosis-associated molecules, tumor necrosis factor-alpha/tumor necrosis factor type-I receptor and Fas/FasL, in sepsis. Chest 125:2238–2246
van Munster BC, Bisschop PH, Zwinderman AH, Korevaar JC, Endert E, Wiersinga WJ, van Oosten HE, Goslings JC, de Rooij SE (2010) Cortisol, interleukins and S100B in delirium in the elderly. Brain Cogn 74:18–23
Tsuruta R, Girard TD, Ely EW, Fujimoto K, Ono T, Tanaka R, Oda Y, Kasaoka S, Maekawa T (2008) Associations between markers of inflammation and cholinergic blockade and delirium in intensive care unit patients: a pilot study. Bull Yamaguchi Med School 55:35–42
Pfister D, Siegemund M, Dell-Kuster S, Smielewski P, Ruegg S, Strebel SP, Marsch SC, Pargger H, Steiner LA (2008) Cerebral perfusion in sepsis-associated delirium. Crit Care 12:R63
Plaschke K, Fichtenkamm P, Schramm C, Hauth S, Martin E, Verch M, Karck M, Kopitz J (2010) Early postoperative delirium after open-heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin-6. Intensive Care Med 36:2081–2089
McGrane S, Girard TD, Thompson JL, Shintani AK, Woodworth A, Ely EW, Pandharipande PP (2011) Procalcitonin and C-reactive protein levels at admission as predictors of duration of acute brain dysfunction in critically ill patients. Crit Care 15:R78
van den Boogaard M, Kox M, Quinn KL, van Achterberg T, van der Hoeven JG, Schoonhoven L, Pickkers P (2011) Biomarkers associated with delirium in critically ill patients and their relation with long-term subjective cognitive dysfunction; indications for different pathways governing delirium in inflamed and noninflamed patients. Crit Care 15:R297
Stellwagen D, Malenka RC (2006) Synaptic scaling mediated by glial TNF-alpha. Nature 440:1054–1059
Esmon CT (2006) Inflammation and the activated protein C anticoagulant pathway. Semin Thromb Hemost 32(Suppl 1):49–60
Gorbacheva L, Pinelis V, Ishiwata S, Strukova S, Reiser G (2010) Activated protein C prevents glutamate- and thrombin-induced activation of nuclear factor-kappaB in cultured hippocampal neurons. Neuroscience 165:1138–1146
Guo H, Liu D, Gelbard H, Cheng T, Insalaco R, Fernandez JA, Griffin JH, Zlokovic BV (2004) Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron 41:563–572
Spapen H, Nguyen DN, Troubleyn J, Huyghens L, Schiettecatte J (2010) Drotrecogin alfa (activated) may attenuate severe sepsis-associated encephalopathy in clinical septic shock. Crit Care 14:R54
Michaluk P, Kaczmarek L (2007) Matrix metalloproteinase-9 in glutamate-dependent adult brain function and dysfunction. Cell Death Differ 14:1255–1258
Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, Huntley GW (2006) Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci 26:1923–1934
Meighan SE, Meighan PC, Choudhury P, Davis CJ, Olson ML, Zornes PA, Wright JW, Harding JW (2006) Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J Neurochem 96:1227–1241
Lorenzl S, Albers DS, Relkin N, Ngyuen T, Hilgenberg SL, Chirichigno J, Cudkowicz ME, Beal MF (2003) Increased plasma levels of matrix metalloproteinase-9 in patients with Alzheimer’s disease. Neurochem Int 43:191–196
Laskowitz DT, Kasner SE, Saver J, Remmel KS, Jauch EC (2009) Clinical usefulness of a biomarker-based diagnostic test for acute stroke: the Biomarker Rapid Assessment in Ischemic Injury (BRAIN) study. Stroke 40:77–85
Vilalta A, Sahuquillo J, Rosell A, Poca MA, Riveiro M, Montaner J (2008) Moderate and severe traumatic brain injury induce early overexpression of systemic and brain gelatinases. Intensive Care Med 34:1384–1392
Izidoro-Toledo TC, Guimaraes DA, Belo VA, Gerlach RF, Tanus-Santos JE (2011) Effects of statins on matrix metalloproteinases and their endogenous inhibitors in human endothelial cells. Naunyn Schmiedebergs Arch Pharmacolo 383:547–554
Morandi A, Hughes CG, Girard TD, McAuley DF, Ely EW, Pandharipande PP (2011) Statins and brain dysfunction: a hypothesis to reduce the burden of cognitive impairment in patients who are critically ill. Chest 140:580–585
Morandi A, Pandharipande PP, Shintani AK, Hughes CG, Vasilevskis EE, Thompson JL, Han JH, Jackson JC, Bernard GR, Laskowitz DT, Ely EW, Girard TD (2012) Statin use and the daily risk of delirium in a prospective cohort of critically ill patients. Am J Respir Crit Care Med 185:A3646
van den Boogaard M, Ramakers BP, van Alfen N, van der Werf SP, Fick WF, Hoedemaekers CW, Verbeek MM, Schoonhoven L, van der Hoeven JG, Pickkers P (2010) Endotoxemia-induced inflammation and the effect on the human brain. Crit Care 14:R81
van Munster BC, Korevaar JC, Zwinderman AH, Levi M, Wiersinga WJ, De Rooij SE (2008) Time-course of cytokines during delirium in elderly patients with hip fractures. J Am Geriatr Soc 56:1704–1709
Marcantonio ER, Rudolph JL, Culley D, Crosby G, Alsop D, Inouye SK (2006) Serum biomarkers for delirium. J Gerontol A Biol Sci Med Sci 61:1281–1286
Chou FP, Chu SC, Cheng MC, Yang SF, Cheung WN, Chiou HL, Hsieh YS (2002) Effect of hemodialysis on the plasma level of type IV collagenases and their inhibitors. Clin Biochem 35:383–388
Perneger TV (1998) What’s wrong with Bonferroni adjustments. BMJ 316:1236–1238
Zampieri FG, Park M, Machado FS, Azevedo LC (2011) Sepsis-associated encephalopathy: not just delirium. Clinics (Sao Paulo) 66:1825–1831
Acknowledgments
Dr. Girard is supported by the National Institutes of Health (NIH) (AG034257) and the Veterans Affairs (VA) Tennessee Valley Geriatric Research, Education, and Clinical Center (GRECC), Dr. Ware is supported by the NIH (HL103836) and an American Heart Association Established Investigator Award, Dr. Pandharipande is supported by the VA Clinical Science Research and Development Service (VA Career Development Award), Dr. Jackson is supported by the NIH (AG031322), and Dr. Ely is supported by the NIH (AG027472 and AG035117) and the VA Tennessee Valley GRECC. Additional funding for this research project was provided by the Saint Thomas Foundation (Nashville, TN, USA), National Institutes of Health (AG001023 and HL007123), Hartford Geriatrics Health Outcomes Research Scholars Award Program, Vanderbilt Physician Scientist Development Program, and Alere Inc. These sponsors had no role in study design; data collection, analysis, and interpretation; or publication of results.
Conflicts of interest
Drs. Girard, Pandharipande, Shintani, and Ely have received honoraria from Hospira Inc. Dr. Pandharipande has received honoraria from Orion Corporation. Drs. Pandharipande and Ely have received grant support from Hospira Inc. Dr. Ely has also received grant support from Eli Lilly and Company and Masimo Corporation and is an advisor to Healthways Inc. All other authors have no disclosures.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Girard, T.D., Ware, L.B., Bernard, G.R. et al. Associations of markers of inflammation and coagulation with delirium during critical illness. Intensive Care Med 38, 1965–1973 (2012). https://doi.org/10.1007/s00134-012-2678-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2678-x