Abstract
Purpose
Emergence of multidrug-resistant strains in intensive care units has renewed interest in colistin, which often remains the only available antimicrobial agent active against resistant Pseudomonas aeruginosa. The aim of this study is to compare lung tissue deposition and antibacterial efficiency between nebulized and intravenous administration of colistin in piglets with pneumonia caused by P. aeruginosa.
Methods
In ventilated piglets, colistimethate was administered 24 h following bronchial inoculation of Pseudomonas aeruginosa (minimum inhibitory concentration of colistin = 2 μg ml−1) either by nebulization (8 mg kg−1 every 12 h, n = 6) or by intravenous infusion (3.2 mg kg−1 every 8 h, n = 6). All piglets were killed 49 h after inoculation. Colistin peak lung tissue concentrations and lung bacterial burden were assessed on multiple post mortem subpleural lung specimens.
Results
Median colistin peak lung concentration following nebulization was 2.8 μg g−1 (25–75% interquartile range = 0.8–13.7 μg g−1). Colistin was undetected in lung tissue following intravenous infusion. In the aerosol group, peak lung tissue concentrations were significantly greater in lung segments with mild pneumonia (median = 10.0 μg g−1, 25–75% interquartile range = 1.8–16.1 μg g−1) than in lung segments with severe pneumonia (median = 1.2 μg g−1, 25–75% interquartile range = 0.5–3.3 μg g−1) (p < 0.01). After 24 h of treatment, 67% of pulmonary segments had bacterial counts <102 cfu g−1 following nebulization and 28% following intravenous administration (p < 0.001). In control animals, 12% of lung segments had bacterial counts <102 cfu g−1 49 h following bronchial inoculation.
Conclusion
Nebulized colistin provides rapid and efficient bacterial killing in ventilated piglets with inoculation pneumonia caused by Pseudomonas aeruginosa.
Similar content being viewed by others
Introduction
Ventilator-associated pneumonia (VAP) is the most frequent nosocomial infection in the critically ill and affects length of stay and cost in the intensive care unit [1, 2]. VAP caused by Pseudomonas aeruginosa is a difficult-to-treat infection associated with high rate of recurrence and frequent selection of new resistance to antibiotics despite adequate initial antimicrobial therapy [3–5]. Emergence of multidrug-resistant strains in intensive care units has renewed interest in an old antibiotic—colistin—which often remains the only available antimicrobial agent active against resistant P. aeruginosa [6, 7].
Colistin is a cationic and multicomponent lipopeptide characterized by rapid bactericidal activities and low levels of resistance [8]. During the past 50 years, however, because of its renal and cochlear toxicities as well as its poor tissue penetration [9, 10], intravenous colistin has been restricted to systemic infections caused by Gram-negative bacteria resistant to all other antimicrobial agents. Both success and failure of the treatment have been reported in a few observational, retrospective or uncontrolled series [11–14]. Lung tissue penetration of colistin following intravenous infusion is unknown, and pharmacokinetic studies have been reported only recently [15, 16]. Nebulization of antibiotics offers the possibility of generating high drug concentrations at the site of infection [17]: administration of aerosolized amikacin or ceftazidime resulted in higher lung deposition and bactericidal effects than intravenous infusion in ventilated piglets with extensive pneumonia [17–20]. Recent clinical use of nebulized colistin suggests good efficiency for treating VAP caused by multidrug-resistant Gram-negative bacteria [21, 22]. It was also reported that a single nebulization of colistin was associated with long-lasting high sputum concentrations in patients with cystic fibrosis [23]. However, the ability of nebulized colistin to reach high lung tissue concentrations in order to provide rapid and efficient bacterial killing has never been investigated.
The aim of this study is to compare lung deposition and bactericidal efficiency of nebulized and intravenous colistin in a porcine model of inoculation pneumonia caused by P. aeruginosa.
Materials and methods
Additional details are provided in the electronic supplement material.
Study design
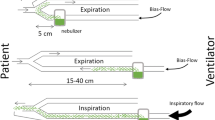
Sixteen Large White−Landrace piglets (20 ± 2 kg) were anesthetized, ventilated (tidal volume = 10 ml kg−1, positive end-expiratory pressure = 5 cmH2O, prone position), and instrumented [18, 19]. In 12 animals, 40 ml suspension containing 106 colony forming units (cfu) ml−1 P. aeruginosa isolated from an immunocompetent patient with VAP (without concurrent bacteremia) was bronchoscopically inoculated in both lungs (10 ml in upper lobes, 10 ml in middle lobes, and 20 ml in lower lobes). Minimum inhibitory concentration (MIC) of colistin was 2 mg l−1. All animals were treated according to guidelines of the Department of Experimental Research of Lille University.
Twenty-four hours later, six piglets received an intravenous dose of 40,000 international units (IU) kg−1 (3.2 mg kg−1) of colistimethate every 8 h using an electrical infuser (intravenous group), and six an aerosol of 100,000 IU kg−1 (8 mg kg−1) of colistimethate diluted in 10 ml sterile water every 12 h, using a vibrating plate nebulizer (aerosol group) (Aeroneb Pro®; Aerogen Ltd., Galway, Ireland). Each administration was performed over 30 min. Nebulization was performed using specific ventilator settings as previously described [20]. One piglet in the intravenous group died of refractory shock after one dose of colistimethate. Mass median aerodynamic diameter of particles (MMAD) was measured using laser velocimeter (Malvern Instrument, Worcestershire, UK) [24]. Extrapulmonary deposition of colistin was determined in vitro as previously described [25]. Blood samples were collected at the end of the antibiotic administration (T0) and then 30 min, 1, 3, 6, and 12 h after T0 in the aerosol group and 30 min, 1, 2, 4, and 8 h in the intravenous group. Animals were killed by exsanguination 1 h after the third aerosol in the aerosol group and 1 h after the fourth intravenous infusion in the intravenous group. Four nontreated inoculated piglets, killed 49 h after inoculation, served as a control group.
Pharmacokinetic and post mortem analysis
Plasma colistin concentrations were measured using high-performance liquid chromatography (HPLC) [26]. The lower limit of quantification for colistin concentration was 0.1 mg l−1. Peak and trough plasma concentrations (C max and C min), elimination half-life (t 1/2), total area under the plasma concentration–time curve (AUC), plasma clearance (CL), and volume of distribution were calculated. Following killing, five “subpleural” specimens were excised from upper, middle, and lower lobes. Each was divided in three blocks. On the first cryomixed and homogenized block, colistin tissue concentrations were measured by HPLC. The second and third blocks served for quantitative bacterial analysis and determination of histological severity (mild or severe pneumonia). The lower limit of quantification for bacterial counts in the lungs was 102 cfu g−1.
Statistical analysis
All data are expressed as mean ± standard deviation (SD) or as median and 25–75% interquartile range (IQR). Colistin peak lung tissue concentrations measured in the aerosol group in different lung segments were compared by one-way analysis of variance for repeated measures followed by post hoc Fisher least significant difference (LSD) test. Comparison of colistin tissue concentrations between severe and mild pneumonias was performed by Mann–Whitney rank-sum test. Lung tissue bacterial burden was compared between groups using Kruskall–Wallis test. Percentage of infected lung segments and lung segments characterized by bacterial counts ranging between 0 and 102 cfu g−1 in the three groups were compared by chi-square test. Pharmacokinetic parameters following the first administration of colistin between the two groups of piglets were compared using bilateral unpaired Student’s test. Statistical analysis was performed by using Sigmastat 9.0 software (SPSS Inc., San Raphael, CA). A p value <0.05 was considered as significant.
Results
Mass median aerodynamic diameter of aerosolized particles
As shown in Table 1, with tidal volume of 300 ml, MMAD (mean ± geometric SD) was 0.99 ± 1.71 μm at the outlet of the nebulizer and 1.00 ± 1.88 μm at the tip of the endotracheal tube. Eighty-four percent of particle sizes at the tip of the endotracheal tube were below 1.9 μm. MMAD was not influenced by increasing tidal volume to 500 ml.
Extrapulmonary deposition of colistin
Of the initial amount of colistimethate inserted into the nebulizer, 3 ± 1% was retained in the nebulizer’s chamber, 26 ± 8% in the inspiratory limb of the respiratory circuit, 1 ± 1% in the endotracheal tube, and 10 ± 3% in the expiratory filter. Total extrapulmonary deposition was 40%. The resulting fraction of colistimethate reaching the respiratory tract was 60% of the initial dose of 8 mg kg−1 placed in the nebulizer chamber, representing a daily dose equivalent to 9.6 mg kg−1 delivered to the respiratory tract.
Colistin lung tissue deposition
Mild pneumonia characterized 52% of secondary pulmonary lobules in the aerosol group versus 73% in the intravenous group. Severe pneumonia characterized 37% of secondary pulmonary lobules in the aerosol group versus 20% in the intravenous group. In the control group, 45% of lung segments were characterized by mild pneumonia and 30% by severe pneumonia.
In the aerosol group, median peak lung tissue concentration of colistin was 2.8 μg g−1 (25–75% IQR: 0.8−13.7 μg g−1). Figure 1 shows the regional distribution of colistin lung tissue concentrations within the infected lung parenchyma in the aerosol group. Colistin peak lung tissue concentrations were significantly lower in lung segments S3 and S10 than in segment S8. Peak lung tissue concentrations were significantly lower in lung areas with severe pneumonia than in lung areas with mild pneumonia (Fig. 2).
Regional distribution of colistin peak lung tissue concentrations measured 1 h after the third aerosol of colistin on lung specimens obtained in different lung segments (S) representative of each lobe: upper lobe (S2), middle lobe (S3), and lower lobe (S6, S8, and S10). MIC Minimal inhibitory concentration. p < 0.05 at the top of the figure indicates statistically significant difference existing between lung segments compared using one-way analysis of variance for repeated measures. Comparisons between two lung segments were performed using post hoc Fisher LSD test: *S3 versus S8, p < 0.05, **S10 versus S8, p < 0.05
Colistin peak lung tissue concentrations according to histological severity of pneumonia following 24-h treatment by nebulized colistin. Left panel shows median and 25–75% interquartile values; right panel shows individual values. Dashed lines represent minimum inhibitory concentration (MIC) of inoculated Pseudomonas aeruginosa. Mild mild pneumonia, Severe severe pneumonia. n number of lung segments in each histological category. Peak colistin lung tissue concentrations were significantly greater in lung segments with mild pneumonia
In the intravenous group, colistin could not be detected in any of the analyzed lung segments.
Bactericidal activity of colistin
After 24 h of colistin administration, median (25–75% IQR) P. aeruginosa lung tissue bacterial burdens in the aerosol, intravenous, and control groups were, respectively, 0 (0–5 × 102) cfu g−1, 4.102 (0–2 × 103) cfu g−1, and 5 × 103 cfu g−1 (5.8 × 102–3.5 × 106) (p < 0.001). As shown in Fig. 3, in the aerosol group, 67% of lung segments were characterized by bacterial counts ranging between 0 and 102 cfu g−1, versus 28% in intravenous and 12% in control group. The difference of segments characterized by bacterial counts ranging between 0 and 102 cfu g−1 was statistically significant between aerosol and intravenous groups and between aerosol and control groups.
Lung bacterial burden of Pseudomonas aeruginosa after 24 h of colistin administration. Lung segments (triangles) were sampled 1 h after the third aerosol in the aerosol group and after the fourth infusion in the intravenous group (IV) and 49 h after the bacterial inoculation in the untreated control group. The grey area indicates the lower limit of quantification for bacterial counts. Asterisk at the top of the figure indicates statistically significant difference existing between the percentage of lung segments characterized by bacterial counts ranging between 0 and 102 cfu g−1 in aerosol and intravenous groups and in aerosol and control groups
Two additional microorganisms were identified in lung tissue specimens: Pasteurella multocida (37% and 36% of lung specimens in aerosol and intravenous groups, respectively) and Streptococcus suis (30% and 20% of lung specimens in aerosol and intravenous groups, respectively). Differences in lung tissue bacterial burden were not statistically significant between the groups.
Serum pharmacokinetics
As shown in Table 2, following the first administration, colistin C max, C min, and AUC were significantly lower and elimination half-life longer in the aerosol group than in the intravenous group. C max/MIC and AUC/MIC ratios were 3.0 ± 0.7 and 6.7 ± 1.0, respectively, in the intravenous piglets. According to a 40% extrapulmonary deposition, 4.8 mg kg−1 colistin entered the lungs by the tracheobronchial tree, whereas in the intravenous group, 3.2 mg kg−1 mg colistin was infused intravenously. Therefore, the antibiotic volume of distribution and plasma clearance corrected for the relative bioavailability of aerosolized colistin were similar in aerosol and intravenous groups.
Discussion
This study, performed in anesthetized and ventilated piglets with inoculation pneumonia caused by P. aeruginosa, shows high colistin lung tissue deposition following nebulization, contrasting with the absence of any detectable deposition following intravenous administration. In the aerosol group, the high lung deposition was associated with rapid and efficient bactericidal activity. Confirming previous experiments on nebulized amikacin [19] and ceftazidime [20], colistin distal lung deposition decreased with severity of pneumonia and aeration loss. Of particular interest, colistin nebulization was associated with decreased systemic exposure compared with intravenous administration.
Lung infection in aerosol and intravenous piglets
Forty-nine hours after inoculation of P. aeruginosa, around 90% of secondary pulmonary lobules were infected in both groups. Two additional microorganisms (P. multocida and S. suis) were isolated from one-third of lung specimens. This result is not surprising, because healthy piglets rapidly develop VAP caused by P. multocida and S. suis after prolonged mechanical ventilation [27, 28]. Therefore, our experimental model is a severe lung infection caused by bronchial inoculation of P. aeruginosa and aggravated by VAP. In both groups, different grades of severity were unevenly distributed through both lungs, with predominance and higher severity in dependent lung segments [27, 28].
Intravenous colistin-induced lung tissue deposition and bactericidal activity
Although recent studies suggest lower toxicity of intravenous colistin than previously reported [14, 29], its clinical use for treating VAP remains controversial. As colistin is a concentration-dependent antibiotic on Gram-negative bacteria, its bactericidal activity depends on peak tissue concentrations reached in infected lung parenchyma [30, 31]. Following three doses of intravenous colistin, lung tissue concentrations were under the detectable threshold in all subpleural specimens, suggesting lack of significant lung deposition. This result is in accordance with a recent study performed in mice with pneumonia caused by multidrug-resistant P. aeruginosa [32]: following intravenous colistin, peak concentrations measured in homogenized lungs were 50-fold less than after intranasal administration, resulting in lack of survival protection. Concentrations measured from homogenized lung represent the total amount of colistin present in interstitial and cell compartments. Pseudomonas aeruginosa does not penetrate into cells and remains in the interstitial space, where colistin exerts its bactericidal activity by binding to bacterial cell membrane. Therefore, colistin concentrations measured from homogenized lung biopsies underestimate “effective” interstitial concentrations, due to a dilution factor caused by intracellular components [33], and the absence of detectable colistin concentration in our study could be partially artifactual. The lack of significant bactericidal effect, however, clearly suggests the lack of significant colistin lung deposition. Another result that may explain the lack of bactericidal effects is the colistin C max/MIC ratio of ~3 in the intravenous group despite high dose of intravenous colistimethate administered. This result confirms data obtained in patients with multidrug-resistant infections (C max/MIC ratio of ~1.5) [34]. Such ratio is inadequate for providing bactericidal effects.
Nebulized colistin-induced lung tissue deposition and bactericidal activity
Most aerosolized particles generated by vibrating-plate nebulizers range between 0.5 and 5 μm, allowing optimal lung deposition. Nebulized colistin is used for treating multidrug-resistant chronic airway infection in patients with cystic fibrosis [35]. In spontaneously breathing patients receiving 2 million IU of nebulized colistin, peak concentrations in sputum were found ten times higher than the colistin MIC breakpoint [36]. Differing from bronchial infection, pneumonia is characterized by distal bronchiolar and alveolar inflammation/infection. In the present study, colistin tissue concentrations were obtained from subpleural lung specimen composed of alveolar structures and distal noncartilaginous bronchioles. Following three consecutive aerosols, peak tissue concentrations were found higher than MIC, indicating significant distal lung deposition. As such concentrations underestimate interstitial concentrations (see above), it is easy to understand why a major bactericidal effect was observed (Fig. 3).
Confirming previous findings, deposition of nebulized colistin was significantly lower in lung segments with severe pneumonia compared with segments with mild pneumonia (Fig. 2). In two dependent lung segments of lower lobe with lung aeration of 3% and 10%, colistin concentrations in lung homogenates were, respectively, 0 and 1.3 μg g−1, likely explaining the lack of bactericidal effect in these segments. Presence of confluent bronchopneumonia with multiple purulent plugs obstructing distal bronchioles markedly reduces lung aeration, thereby explaining decreased colistin lung deposition. Interestingly, the bactericidal effect was observed in 7 of 13 lung segments with severe pneumonia despite more limited distal lung tissue deposition. Very likely, the presence of intraparenchymal pseudocysts and bronchiolar distension in lung areas with severe pneumonia represents one of the routes by which nebulized colistin reaches distal infected lung [19, 37].
Contrasting with previous data showing that nebulization of amikacin does not decrease systemic exposure and risk of toxicity in piglets with severe lung infection [19], colistin plasma concentrations after nebulization were significantly lower than after intravenous administration (Table 2). Colistimethate, which is negatively charged at body pH, is likely confronted with alveolar basement membrane formed also by negative charges [38, 39]. Both electrical charges resulted in slow systemic passage of colistin through alveolar–capillary barrier and therefore increased the colistin elimination half-life in the aerosol group. The smaller AUC also suggests decreased exposure to colistin systemic toxicity. These results confirm a recent study performed in patients with cystic fibrosis treated by inhaled colistin and reporting reduced systemic exposure [23]. In the present study, significant lower concentrations were found in lung areas with severe pneumonia despite the administration of high doses of aerosolized colistimethate (Fig. 2). This finding indicates that an aerosol dose of 8 mg kg−1 every 12 h, a dosage exceeding by threefold the dose commonly reported in the literature [21], is required for efficient treatment of pneumonia caused by sensitive P. aeruginosa. Moreover, the significantly reduced systemic exposure resulting from nebulized colistin compared with intravenous colistin provides the possibility to increase doses without increasing risk of toxicity.
Limitations of the study
Available therapeutic options for treating VAP caused by multidrug-resistant Gram-negative bacteria are limited. Although recent, uncontrolled studies have reported clinical efficiency of nebulized and intravenous colistin, these two routes of administration have never been compared [40, 41]. Our study, performed in a highly relevant animal model close to human VAP, evidences the superiority of nebulized colistin compared with intravenous colistin as far as lung deposition, bactericidal activity, and systemic toxicity are concerned. However, one should remember that the tracheobronchial tree anatomy of ventilated humans in the supine position is different from that of piglets in the prone position (Fig. 4). As a consequence, the encouraging results obtained from this experimental study cannot be automatically extrapolated to patients with VAP. Another limitation is that nebulized colistin is unable to treat pneumonia when associated with bacteremia.
Many clinicians believe that aerosol therapy as adjunctive to intravenous therapy offers an attractive alternative to intravenous or nebulized therapy alone for treating VAP. The hypothesis that a combination of nebulized and intravenous antibiotics could increase lung tissue deposition and accelerate bacterial killing was previously tested in four experimental piglets whose lungs were infected by massive bronchial inoculation of Escherichia coli and that received a combination of nebulized and intravenous amikacin (unpublished data that were part of a previously published study [19]). Unfortunately, no additional increase in lung tissue concentrations were evidenced, whereas increased trough systemic concentrations were observed, increasing the risk of systemic toxicity. In this experimental study, very high amikacin concentrations were found in lymphatic vessels, suggesting large absorption of amikacin into lymphatic vessels of lung interstitial space. It has to be pointed out that intravenous amikacin, in contrast to colistin, diffuses into the alveolar space and that nebulized amikacin diffuses into the systemic circulation in presence of lung infection. The present study provides solid evidence that lung deposition of intravenous colistin is extremely reduced, if not null. Therefore, it is hazardous to expect that combining nebulized and intravenous colistin might increase colistin lung tissue concentrations. In contrast, increased systemic concentrations resulting from intravenous administration may increase risk of renal toxicity. Intravenous colistin, unlike aminoglycosides and beta-lactams, does not cross the alveolar–capillary barrier of the infected lung parenchyma. Therefore, the rationale for combining nebulized and intravenous colistin as treatment for VAP appears weak. In fact, there is a strong rationale for treating bacteremic VAP caused by resistant P. aeruginosa or Acinetobacter baumanii with a combination of nebulized and intravenous colistin. Such a combination remains the only therapeutic option. Unfortunately, our model of inoculation pneumonia is not associated with positive blood cultures and does not allow the assessment of this specific issue. Another experimental study assessing the effects of combined intravenous and nebulized colistin would certainly provide relevant information regarding treatment of pneumonia associated with bacteremia.
In conclusion, nebulized colistin provides rapid and efficient bacterial killing in ventilated piglets with inoculation pneumonia caused by P. aeruginosa. Further randomized and comparative clinical studies are required to assess the efficiency of nebulized colistin for treating VAP caused by multidrug-resistant Gram-negative pathogens.
References
Rouby JJ (1996) Nosocomial infection in the critically ill: the lung as a target organ. Anesthesiology 84:757–759
Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, Wolff M, Spencer RC, Hemmer M (1995) The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA 274:639–644
Rello J, Mariscal D, March F, Jubert P, Sanchez F, Valles J, Coll P (1998) Recurrent Pseudomonas aeruginosa pneumonia in ventilated patients: relapse or reinfection? Am J Respir Crit Care Med 157:912–916
Crouch Brewer S, Wunderink RG, Jones CB, Leeper KV Jr (1996) Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest 109:1019–1029
Mesaros N, Nordmann P, Plesiat P, Roussel-Delvallez M, Van Eldere J, Glupczynski Y, Van Laethem Y, Jacobs F, Lebecque P, Malfroot A et al (2007) Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin Microbiol Infect 13:560–578
Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL (2006) Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 6:589–601
Falagas ME, Kasiakou SK (2005) Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis 40:1333–1341
Evans ME, Feola DJ, Rapp RP (1999) Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann Pharmacother 33:960–967
Wolinsky E, Hines JD (1962) Neurotoxic and nephrotoxic effects of clolistin in patients with renal disease. N Engl J Med 266:759–762
Elwood CM, Lucas GD, Muehrcke RC (1966) Acute renal failure associated with sodium colistimethate treatment. Arch Intern Med 118:326–334
Kallel H, Hergafi L, Bahloul M, Hakim A, Dammak H, Chelly H, Hamida CB, Chaari A, Rekik N, Bouaziz M (2007) Safety and efficacy of colistin compared with imipenem in the treatment of ventilator-associated pneumonia: a matched case–control study. Intensive Care Med 33:1162–1167
Garnacho-Montero J, Ortiz-Leyba C, Jimenez-Jimenez FJ, Barrero-Almodovar AE, Garcia-Garmendia JL, Bernabeu-Wittel IM, Gallego-Lara SL, Madrazo-Osuna J (2003) Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin Infect Dis 36:1111–1118
Fica CA, Cespedes JI, Gompertz GM, Jalon VM, Sakurada ZA, Saez LE (2007) Intravenous colistin in the treatment of infections due to pan-resistant Gram negative bacilli. Rev Chilena Infectol 24:360–367
Falagas ME, Rafailidis PI, Kasiakou SK, Hatzopoulou P, Michalopoulos A (2006) Effectiveness and nephrotoxicity of colistin monotherapy vs. colistin-meropenem combination therapy for multidrug-resistant Gram-negative bacterial infections. Clin Microbiol Infect 12:1227–1230
Poudyal A, Howden BP, Bell JM, Gao W, Owen RJ, Turnidge JD, Nation RL, Li J (2008) In vitro pharmacodynamics of colistin against multidrug-resistant Klebsiella pneumoniae. J Antimicrob Chemother 62:1311–1318
Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A et al (2009) Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother 53:3430–3436
Rouby JJ, Goldstein I, Lu Q (2006) Inhaled antibiotic therapy. In: Tobin MJ (ed) Principles and practice of mechanical ventilation, Chapter 64, 2nd edn. McGraw-Hill, New York, pp 1311–1332
Tonnellier M, Ferrari F, Goldstein I, Sartorius A, Marquette CH, Rouby JJ (2005) Intravenous versus nebulized ceftazidime in ventilated piglets with and without experimental bronchopneumonia: comparative effects of helium and nitrogen. Anesthesiology 102:995–1000
Goldstein I, Wallet F, Nicolas-Robin A, Ferrari F, Marquette CH, Rouby JJ (2002) Lung deposition and efficiency of nebulized amikacin during Escherichia coli pneumonia in ventilated piglets. Am J Respir Crit Care Med 166:1375–1381
Ferrari F, Lu Q, Girardi C, Petitjean O, Marquette CH, Wallet F, Rouby JJ (2009) Nebulized ceftazidime in experimental pneumonia caused by partially resistant Pseudomonas aeruginosa. Intensive Care Med 35:1792–1800
Michalopoulos A, Kasiakou SK, Mastora Z, Rellos K, Kapaskelis AM, Falagas ME (2005) Aerosolized colistin for the treatment of nosocomial pneumonia due to multidrug-resistant Gram-negative bacteria in patients without cystic fibrosis. Crit Care 9:R53–R59
Kwa AL, Loh C, Low JG, Kurup A, Tam VH (2005) Nebulized colistin in the treatment of pneumonia due to multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Clin Infect Dis 41:754–757
Ratjen F, Rietschel E, Kasel D, Schwiertz R, Starke K, Beier H, van Koningsbruggen S, Grasemann H (2006) Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J Antimicrob Chemother 57:306–311
Goldstein I, Wallet F, Robert J, Becquemin MH, Marquette CH, Rouby JJ (2002) Lung tissue concentrations of nebulized amikacin during mechanical ventilation in piglets with healthy lungs. Am J Respir Crit Care Med 165:171–175
Ferrari F, Liu ZH, Lu Q, Becquemin MH, Louchahi K, Aymard G, Marquette CH, Rouby JJ (2008) Comparison of lung tissue concentrations of nebulized ceftazidime in ventilated piglets: ultrasonic versus vibrating plate nebulizers. Intensive Care Med 34:1718–1723
Le Brun PP, de Graaf AI, Vinks AA (2000) High-performance liquid chromatographic method for the determination of colistin in serum. Ther Drug Monit 22:589–593
Marquette CH, Wermert D, Wallet F, Copin MC, Tonnel AB (1999) Characterization of an animal model of ventilator-acquired pneumonia. Chest 115:200–209
Sartorius A, Lu Q, Vieira S, Tonnellier M, Lenaour G, Goldstein I, Rouby JJ (2007) Mechanical ventilation and lung infection in the genesis of air-space enlargement. Crit Care 11:R14
Reina R, Estenssoro E, Saenz G, Canales HS, Gonzalvo R, Vidal G, Martins G, Das Neves A, Santander O, Ramos C (2005) Safety and efficacy of colistin in Acinetobacter and Pseudomonas infections: a prospective cohort study. Intensive Care Med 31:1058–1065
Gunderson BW, Ibrahim KH, Hovde LB, Fromm TL, Reed MD, Rotschafer JC (2003) Synergistic activity of colistin and ceftazidime against multiantibiotic-resistant Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 47:905–909
Li J, Coulthard K, Milne R, Nation RL, Conway S, Peckham D, Etherington C, Turnidge J (2003) Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. J Antimicrob Chemother 52:987–992
Aoki N, Tateda K, Kikuchi Y, Kimura S, Miyazaki C, Ishii Y, Tanabe Y, Gejyo F, Yamaguchi K (2009) Efficacy of colistin combination therapy in a mouse model of pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. J Antimicrob Chemother 63:534–542
Nix DE, Goodwin SD, Peloquin CA, Rotella DL, Schentag JJ (1991) Antibiotic tissue penetration and its relevance: impact of tissue penetration on infection response. Antimicrob Agents Chemother 35:1953–1959
Markou N, Markantonis SL, Dimitrakis E, Panidis D, Boutzouka E, Karatzas S, Rafailidis P, Apostolakos H, Baltopoulos G (2008) Colistin serum concentrations after intravenous administration in critically ill patients with serious multidrug-resistant, gram-negative bacilli infections: a prospective, open-label, uncontrolled study. Clin Ther 30:143–151
Ryan G, Mukhopadhyay S, Singh M (2000) Nebulised anti-pseudomonal antibiotics for cystic fibrosis. Cochrane Database Syst Rev CD001021
MacGowan AP, Wise R (2001) Establishing MIC breakpoints and the interpretation of in vitro susceptibility tests. J Antimicrob Chemother 48(suppl 1):17–28
Elman M, Goldstein I, Marquette CH, Wallet F, Lenaour G, Rouby JJ (2002) Influence of lung aeration on pulmonary concentrations of nebulized and intravenous amikacin in ventilated piglets with severe bronchopneumonia. Anesthesiology 97:199–206
Barrowcliffe MP, Jones JG (1987) Solute permeability of the alveolar capillary barrier. Thorax 42:1–10
Brody JS, Vaccaro CA, Hill NS, Rounds S (1984) Binding of charged ferritin to alveolar wall components and charge selectivity of macromolecular transport in permeability pulmonary edema in rats. Circ Res 55:155–167
Falagas ME, Siempos II, Rafailidis PI, Korbila IP, Ioannidou E, Michalopoulos A (2009) Inhaled colistin as monotherapy for multidrug-resistant gram (−) nosocomial pneumonia: a case series. Respir Med 103:707–713
Michalopoulos A, Fotakis D, Virtzili S, Vletsas C, Raftopoulou S, Mastora Z, Falagas ME (2008) Aerosolized colistin as adjunctive treatment of ventilator-associated pneumonia due to multidrug-resistant Gram-negative bacteria: a prospective study. Respir Med 102:407–412
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Girardi: Recipient of a scholarship provided by the Ministère Français des Affaires Etrangères (ref. 315372K).
This article is discussed in the editorial available at: doi:10.1007/s00134-010-1883-8.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, Q., Girardi, C., Zhang, M. et al. Nebulized and intravenous colistin in experimental pneumonia caused by Pseudomonas aeruginosa . Intensive Care Med 36, 1147–1155 (2010). https://doi.org/10.1007/s00134-010-1879-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1879-4