Abstract
Introduction
To determine the effects of tranexamic acid (TXA) on transfusions in patients undergoing hip replacement with a hybrid or cementless prosthesis.
Methods
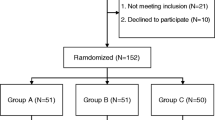
A group of 172 consecutive patients aged 18 years or older who underwent elective hip replacement with uncemented or hybrid prostheses, undergoing surgery between January 2012 and January 2014 by the same primary surgeon and anesthesiologist, were retrospectively included. TXA (1 g) was administered immediately before incision in the TXA group. Primary variables included number of red blood cell transfusions and the influence of TXA for each type of prosthesis. Secondary variables included hematocrit at discharge, length of hospital stay, thrombosis or pulmonary embolism, seizures, and death.
Results
Average transfusion was 1.53 units/patient in the control group compared to 0.6 units/patient in the TXA group (z = 6.29; U = 1640.5; p < 0.0001). TXA use was significantly correlated with the number of units transfused (p < 0.0001, 95% CI −1.24 to −0.68). Odds risk reduction for transfusion was observed during surgery (OR: 0.14; CI 0.06–0.29; p < 0.0001) and during the rest of hospital stay (OR: 0.11; CI 0.01–0.96; p = 0.046). Both hybrid and cementless prostheses that received TXA were transfused less than control groups (0.57 ± 1 vs. 1.7 ± 1 p < 0.01 and 0.65 ± 1 vs. 1.24 ± 1 p < 0.01). No difference was observed between the groups regarding adverse effects. Hematocrit values at discharge and length of hospital stay were similar between groups. No deaths were observed during hospital stay.
Conclusions
TXA reduced transfusions without increasing the prevalence of adverse effects. This reduction was observed during surgery and the following days of hospital stay for both for hybrid and cementless prosthesis.
Zusammenfassung
Einleitung
Die Auswirkungen von Tranexamsäure (TXA) auf die Transfusionen bei Patienten, die sich einer Operation zur Implantation einer Hybrid- oder zementfreien Endoprothese unterzogen, werden bestimmt.
Methoden
Eine Gruppe von 172 konsekutiven Patienten ≥18 Jahren, die zwischen Januar 2012 und Januar 2014 im Rahmen einer Hüftoperation, die vom selben Chirurgen und Anästhesisten durchgeführt wurde, eine zementfreie oder Hybridendoprothese erhielten, wurden retrospektiv eingeschlossen. Die TXA (1 g) wurde in der TXA-Gruppe unmittelbar vor der Inzision injiziert. Die primären Variablen waren die Anzahl der übertragenen roten Blutkörperchen sowie der Einfluss von TXA auf jede Endoprothesenform. Die sekundären Variablen waren Hämatokrit bei Entlassung aus dem Krankenhaus, Hospitalisationsdauer, Thrombose oder Lungenembolie, Epilepsie und Tod.
Ergebnisse
Die durchschnittliche Transfusionsmenge betrug 1,53 Einheiten/Patient in der Kontrollgruppe verglichen mit 0,6 Einheiten/Patient in der TXA-Gruppe (z = 6,29; U = 1640,5; p < 0,0001). TXA korrelierte signifikant mit der Anzahl der übertragenen Einheiten (p < 0,0001; 95% CI −1,24 und −0,68). Während der Operation wurde eine Reduzierung des Odds-Ratios beobachtet (OR: 0,14; 95% CI 0,06–0,29; p < 0,0001) sowie auch während der übrigen Hospitalisationsdauer (OR: 0,11; 95% CI 0,01–0,96; p = 0,046). Sowohl in der Gruppe mit Hybridendoprothese als auch in der Gruppe mit zementfreier Endoprothese, die beide TXA erhalten hatten, waren weniger Transfusionen nötig als bei den Kontrollgruppen (0,57 ± 1 vs. 1,7 ± 1; p < 0,01 und 0,65 ± 1 vs. 1,24 ± 1; p < 0,01). Hinsichtlich der unerwünschten Ereignisse wurde kein Unterschied zwischen den Gruppen festgestellt. Die Hämatokritwerte bei Entlassung und die Hospitalisationsdauer waren in beiden Gruppen ähnlich. Todesfälle während des Krankenhausaufenthalts gab es keine.
Schlussfolgerung
TXA reduzierte die Transfusionen, ohne das Auftreten unerwünschter Ereignisse zu erhöhen. Diese Reduzierung wurde während der Operation und in den folgenden Tagen des Krankhausaufenthalts sowohl in der Gruppe mit Hybridendoprothese als auch in der Gruppe mit zementfreier Endoprothese beobachtet.


Similar content being viewed by others
References
Taylor SE, Cross MH (2013) Clinical strategies to avoid blood transfusion. Anaesth Intensive Care Med 14:48–50. doi:10.1016/j.mpaic.2012.11.012
Barrachina B, Lopez-Picado A, Remon M et al (2016) Tranexamic acid compared with placebo for reducing total blood loss in hip replacement surgery. Anesth Analg 122:986–995. doi:10.1213/ANE.0000000000001159
Poeran J, Rasul R, Suzuki S et al (2014) Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ 349:g4829. doi:10.1136/bmj.g4829
Kim TK, Chang CB, Koh IJ (2014) Practical issues for the use of tranexamic acid in total knee arthroplasty: a systematic review. Knee Surg Sports Traumatol Arthrosc 22:1849–1858. doi:10.1007/s00167-013-2487-y
Sukeik M, Alshryda S, Haddad FS, Mason JM (2011) Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br 93:39–46. doi:10.1302/0301-620X.93B1.24984
Banerjee S, Issa K, Pivec R et al (2013) Intraoperative pharmacotherapeutic blood management strategies in total knee arthroplasty. J Knee Surg 26:379–385. doi:10.1055/s-0033-1353992
Mak JCS, Fransen M, Jennings M et al (2012) Evidence-based review for patients undergoing elective hip and knee replacement. ANZ J Surg 84:17–24. doi:10.1111/ans.12109
Memtsoudis SG, Bs MH, Russell LA et al (2013) Consensus statement from the consensus conference on bilateral total knee arthroplasty group. Clin Orthop Relat Res 471:2649–2657. doi:10.1007/s11999-013-2976-9
March GM, Elfatori S, Beaulé PE (2013) Clinical experience with tranexamic acid during primary total hip arthroplasty. Hip Int 23:72–79. doi:10.5301/HIP.2013.10724
Zhang Y, Zhang L, Ma X et al (2016) What is the optimal approach for tranexamic acid application in patients with unilateral total hip arthroplasty? Orthopäde. doi:10.1007/s00132-016-3252-y
Phan DL (2015) Can tranexamic acid change preoperative anemia management during total joint arthroplasty? World J Orthop 6:521. doi:10.5312/wjo.v6.i7.521
Machin JT, Batta V, Soler JA et al (2014) Comparison of intra-operative regimes of tranexamic acid administration in primary total hip replacement. Acta Orthop Belg 80:228–233
Clarke AM, Dorman T, Bell MJ (1992) Blood loss and transfusion requirements in total joint arthroplasty. Ann R Coll Surg Engl 74:360–363
Trice ME, Walker RH, D’Lima DD et al (1999) Blood loss and transfusion rate in noncemented and cemented/hybrid total hip arthroplasty. Is there a difference? A comparison of 25 matched pairs. Orthopedics 22:s141–s144. doi:10.1007/s00776-008-1317-4
Rajesparan K, Biant LC, Ahmad M, Field RE (2009) The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br 91:776–783. doi:10.1302/0301-620X.91B6.22393
Xie J, Ma J, Yue C et al (2016) Combined use of intravenous and topical tranexamic acid following cementless total hip arthroplasty: a randomised clinical trial. Hip Int 26:36–42. doi:10.5301/hipint.5000291
Sano M, Hakusui H, Kojima C, Akimoto T (1976) Absorption and excretion of tranexamic acid following intravenous, intramuscular and oral administrations in healthy volunteers. Rinsho Yakuri Jpn J Clin Pharmacol Ther 7:375–382. doi:10.3999/jscpt.7.375
Benoni G, Carlsson A, Petersson C, Fredin H (1995) Does tranexamic acid reduce blood loss in knee arthroplasty? Am J Knee Surg 8:88–92. doi:10.1017/CBO9781107415324.004
Husted H, Blønd L, Sonne-Holm S et al (2003) Tranexamic acid reduces blood loss and blood transfusions in primary total hip arthroplasty: a prospective randomized double-blind study in 40 patients. Acta Orthop Scand 74:665–669. doi:10.1080/00016470310018171
Yamasaki S, Masuhara K, Fuji T (2005) Postoperative blood loss in cementless total hip arthroplasty. J Bone Joint Surg Am 87–A:766–770
Ho KM, Ismail H (2003) Use of intravenous tranexamic acid to reduce allogeneic blood transfusion in total hip and knee arthroplasty: a meta-analysis. Anaesth Intensive Care 31:529–537
Lindoff C, Rybo G, Astedt B (1993) Treatment with tranexamic acid during pregnancy, and the risk of thrombo-embolic complications. Thromb Haemost 70:238–240
Gandhi R, Evans HMK, Mahomed SR, Mahomed NN (2013) Tranexamic acid and the reduction of blood loss in total knee and hip arthroplasty: a meta-analysis. BMC Res Notes 6:184. doi:10.1186/1756-0500-6-184
Lin ZX, Woolf SK (2016) Safety, efficacy, and cost-effectiveness of tranexamic acid in orthopedic surgery. Orthopedics 39:119–130. doi:10.3928/01477447-20160301-05
Gillette BP, DeSimone LJ, Trousdale RT et al (2013) Low risk of thromboembolic complications with tranexamic acid after primary total hip and knee arthroplasty. Clin Orthop Relat Res 471:150–154. doi:10.1007/s11999-012-2488-z
Bell D, Marasco S, Almeida A, Rowland M (2010) Tranexamic acid in cardiac surgery and postoperative seizures: a case report series. Heart Surg Forum 13:E257–9. doi:10.1532/HSF98.20101014
Murkin JM, Falter F, Granton J et al (2010) High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg 110:350–353. doi:10.1213/ANE.0b013e3181c92b23
Kratzer S, Irl H, Mattusch C et al (2014) Tranexamic acid impairs γ‑aminobutyric acid receptor type a‑mediated synaptic transmission in the murine amygdala: a potential mechanism for drug-induced seizures? Anesthesiology 120:639–649. doi:10.1097/ALN.0000000000000103
Cordero-Ampuero J, De Dios M (2010) What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin Orthop Relat Res 468:3268–3277. doi:10.1007/s11999-010-1411-8
Saleh K, Olson M, Resig S et al (2002) Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res 20:506–515. doi:10.1016/S0736-0266(01)00153-X
Noailles T, Brulefert K, Chalopin A et al (2015) What are the risk factors for post-operative infection after hip hemiarthroplasty? Systematic review of literature. Int Orthop. doi:10.1007/s00264-015-3033-y
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Fígar, S. Mc Loughlin, P.A. Slullitel, W. Scordo, and M.A. Buttaro declare that they have no competing interests.
The present study was approved by the Ethics Board of our institution “Comité de Etica de Protocolos de Investigación (CEPI)”; “Hospital Italiano de Buenos Aires”. The present study was waived from informed consent by the Ethics Review Board due to its retrospective design. All details that might disclose the identity of the subjects under study have been omitted.
Additional information
Authors contribution: Fígar A, Mc Loughlin S, and Slullitel PA designed the research, analyzed the data, and wrote the manuscript; Scordo W provided data from the blood bank and transfusions; Buttaro MA performed the surgeries and overviewed analysis and final manuscript.
Rights and permissions
About this article
Cite this article
Fígar, A., Mc Loughlin, S., Slullitel, P.A. et al. Influence of single-dose intravenous tranexamic acid on total hip replacement. Orthopäde 46, 359–365 (2017). https://doi.org/10.1007/s00132-016-3352-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00132-016-3352-8