Abstract
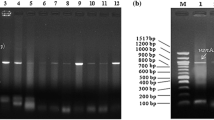
Due to the widespread occurrence of mecA-encoded methicillin resistance in Staphylococcus aureus (MRSA), treatment of staphylococcal infections is shifted to glycopeptide antibiotics like vancomycin and teicoplanin. The selective pressure of glycopeptides has eventually led to the emergence of staphylococci with increased resistance. Of great concern is vanA-encoded high level vancomycin and teicoplanin resistance in MRSA (VRSA). Therefore, this study aimed at investigating the occurrence of VRSA in surface waters. Out of 290, two staphylococcal isolates identified as MRSA Al11, Ba01, and one as MRS Co11 through 16S rRNA sequencing, also displayed high level resistance towards vancomycin and teicoplanin. These staphylococcal isolates were found to harbor vanA gene with sequence similarities of 99 %–100 % to the vanA gene extracted from vancomycin- and teicoplanin-resistant enterococcal (VRE) surface water isolates of Enterococcus faecalis Cr07, E07, Pb06 and E. faecium E330. High level glycopeptide resistance rendering protein encoded by the vanA gene, d-alanine–d-lactate ligase found in VRE, was also shown to be present in all vanA-type staphylococcal isolates through western blot. Current study elucidated that surface waters provide high potential for enterococcal vanA gene being transferred to MRSA, so called VRSA, and require special scientific consideration.




Similar content being viewed by others
References
Aktan Y, Tan S, Icgen B (2013) Characterization of lead-resistant river isolate Enterococcus faecalis and assessment of its multiple metal and antibiotic resistance. Environ Monitor Assess 185:5285–5293
Arthur M, Molinas C, Depardieu F, Courvalin P (1993) Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol 175:117–127
Arthur M, Reynolds P, Courvalin P (1996) Glycopeptide resistance in enterococci. Trends Microbiol 4:401–407
Brown DFJ, Edwards DI, Hawkey PM, Morrison D, Ridgway GL, Towner KJ (2005) Guidelines for the laboratory diagnosis and susceptibility testing of methicillin-resistant Staphylococcus aureus (MRSA). J Antimicrob Chemother 56:1000–1018
Clinical and Laboratory Standards Institute, CLSI (2006) Performance Standards for Antimicrobial Susceptibility Testing. CLSI approved standard M100eS16
Clinical and Laboratory Standards Institute, CLSI (2012) Performance Standards for Antimicrobial Susceptibility Testing; 22nd Informational Supplement M100-S13. National Committee for Clinical Laboratory Standards, Villanova
Cooper MA, Fiorini MT, Abell C, Williams DH (2000) Binding of vancomycin group antibiotics to d-alanine and d-lactate presenting self-assembled monolayers. Bioorg Med Chem 8:2609–2616
Courvalin P (2006) Vancomycin resistance in gram-positive cocci. Clin Infect Dis 42:25–34
Handwerger S, Pucci MJ, Volk KJ, Liu J, Lee MS (1994) Vancomycin-resistant Leuconostoc mesenteroides and Lactobacillus casei synthesize cytoplasmic peptidoglycan precursors that terminate in lactate. J Bacteriol 176:260–264
Handwerger S, Skoble J, Discotto LF, Pucci MJ (1995) Heterogeneity of the vanA gene in clinical isolates of enterococci from the Northeastern United States. Antimicrob Agents Chemother 39:362–368
Howden BP, Davies JK, Johnson PDR et al (2010) Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and eterogeneous vancomycin- intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev 23:99–138
Hunter PA, Dawson S, French GL, Goossens H, Hawkey PM, Kuijper EJ, Nathwani D, Taylor DJ, Teale CJ, Warren RE, Wilcox MH, Woodford N, Wulf MW, Piddock LJ (2010) Antimicrobial-resistant pathogens in animal and man: prescribing, practices and policies. J Antimicrob Chemother 65:13–17
Icgen B, Yilmaz F (2014) Co-occurrence of antibiotic and heavy metal resistance in Kızılırmak river isolates. Bull Environ Contam Toxicol 93:735–743
Katayama Y, Ito T, Hiramatsu K (2000) A new class of genetic element, Staphylococcus Cassette Chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 44:1549–1555
Ke D, Picard FJ, Martineau F, Ménard C, Roy PH, Ouellette M, Bergeron MG (1999) Development of a PCR assay for rapid detection of enterococci. J Clin Microbiol 37:3497–3503
Kim C, Milheirico C, Gardete S, Holmes M, Holden MTG, de Lencastre H, Tomasz A (2012) Properties of a novel PBP2A protein homologue from Staphylococcus aureus strain LGA251 and its contribution to the β-lactam resistant phenotype. J Biol Chem 287:36854–36863
Lane DJ, Pace B, Olsen GJ, Stahl DA, Sogin ML, Pace NR (1985) Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci 82:6955–6959
Luczkiewicz A, Jankowska K, Fudala-Ksiazek S, Olańczuk-Neyman K (2010) Antimicrobial resistance of fecal indicators in municipal wastewater treatment plant. Water Res 44:5089–5097
Mellmann A, Becker K, von Eiff C, Keckevoet U, Schumann P, Harmsen D (2006) Sequencing and staphylococci identification. Emerg Infect Dis 12:333–336
Miele A, Bandera M, Goldstein BP (1995) Use of primers selective for vancomycin resistance genes to determine van genotype in enterococci and to study gene organization in vanA isolates. Antimicrob Agents Chemother 39:1772–1778
Młynarczyk A, Młynarczyk G, Łuczak M (2002) Conjugative transfer of glycopeptide and macrolide resistant genes among enterococci and from Enterococcus faecalis to Staphylococcus aureus. Med Dosw Mikrobiol 54:21–28
Morris D, Galvin S, Boyle F, Hickey P, Mulligan M, Cormican M (2012) Enterococcus faecium of the vanA genotype in rural drinking water, effluent, and the aqueous environment. Appl Environ Microbiol 78:596–598
Noble WC, Virani Z, Cree RG (1992) Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol Lett 72:195–198
Périchon B, Courvalin P (2000) Update on vancomycin resistance. Int J Clin Pract 54:250–254
Périchon B, Courvalin P (2009) VanA-type vancomycin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 53:4580–4587
Perl TM (1999) The threat of vancomycin resistance. Am J Med 106:26S–37S
Ranotkar S, Kumar P, Zutshi S, Prashanth KS, Bezbaruah B, Anand J, Lahkar M (2014) Vancomycin-resistant enterococci: troublemaker of the 21st century. J Glob Antimicrob Resist 2:205–212
Reynolds PE (1989) Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur J Clin Microbiol Infect Dis 8:943–950
Schweiger ES, Scheinfeld NS, Tischler HR, Weinberg JM (2003) Linezolid and quinupristin/dalfopristin: novel antibiotics for Gram-positive infections of the skin. J Drugs Dermatol 2:378–383
Seyedmonir E, Yilmaz F, Icgen B (2015) mecA gene dissemination among staphylococcal and non-staphylococcal isolates shed in surface waters. Bull Environ Contam Toxicol 95:131–138
Sletvold H, Johnsen PJ, Simonsen GS, Aasnaes B, Sundsfjord A, Nielsen KM (2007) Comparative DNA analysis of two vanA plasmids from Enterococcus faecium strains isolated from poultry and a poultry farmer in Norway. Antimicrob Agents Chemother 51:736–739
Sletvold H, Johnsen PJ, Wikmark OG, Simonsen GS, Sundsfjord A, Nielsen KM (2010) Tn1546 is part of a larger plasmid-encoded genetic unit horizontally disseminated among clonal Enterococcus faecium lineages. J Antimicrob Chemother 65:1894–1906
Stalder T, Barraud O, Jové T, Casellas M, Gaschet M, Dagot C et al (2014) Quantitative and qualitative impact of hospital effluent on dissemination of the integron pool. ISME J 8:768–777
Sun W, Chen H, Liu Y, Zhao C, Nichols WW, Chen M et al (2009) Prevalence and characterization of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates from 14 cities in China. Antimicrob Agents Chemother 53:3642–3649
Weigel LM, Donlan RM, Shin DH, Jensen B, Clark NC, McDougal LK, Zhu W, Musser KA, Thompson J et al (2007) High-level vancomycin-resistant Staphylococcus aureus isolates associated with a polymicrobial biofilm. Antimicrob Agents Chemother 51:231–238
Werner G, Dahl KH, Willems RJ (2006) Composite elements encoding antibiotic resistance in Enterococcus faecium and Enterococcus faecalis. In: Kobayashi N (ed) Drug resistance in Enterococci: epidemiology and molecular markers. Research Signpost, Fort PO, Trivandrum, pp 157–208
Whitener CJ, Park SY, Browne FA, Parent LJ, Julian K, Bozdogan B et al (2004) Vancomycin-resistant Staphylococcus aureus in the absence of vancomycin exposure. Clin Infect Dis 38:1049–1055
Willems RJ, Top J, van den Braak N, van Belkum A, Mevius DJ, Hendriks G, van Santen-Verheuvel M, van Embden JD (1999) Molecular diversity and evolutionary relationships of Tn1546-like elements in enterococci from humans and animals. Antimicrob Agents Chemother 43:483–491
Zhu W, Clark NC, McDougal LK, Hageman J, McDonald LC, Patel JB (2008) Vancomycin-resistant Staphylococcus aureus isolates associated with Inc18-like vanA plasmids in Michigan. Antimicrob Agents Chemother 52:452–457
Acknowledgments
This work was supported by TUBITAK-Turkey through both Projects 1001 and 1002 with the Project Numbers of 113Z198 and 114Z973, respectively.