Abstract
The main aim of this study was to evaluate the toxicity of different poultry manure landfill leachates, using a well-known toxicity test system (MS3). The bioassay was made using a battery of toxicity tests including acute toxicity with crustacean (Daphnia magna), algae (Chlorella vulgaris) and the in vitro toxicity test with the fish cell line RTG-2. On D. magna was high mortality for zero time and almost 100 % and 70 %–80 % mortality for sawdust and straw poultry manure respectively. No effects on C. vulgaris, was observed after the leachate exposure. None of the parameters considered: protein, EROD activity, β-gal activity and neutral red, showed differences between control test and the leachate collected from exposure to poultry manure.
Similar content being viewed by others
The utilization of poultry manure as an organic fertilizer is essential in improving soil productivity and crop production (Dikinya and Mufwanzala 2010). While the use of organic wastes as manure has been in practice for centuries world-wide and in the recent times (López-Masquera et al. 2008), there still exists a need to assess the potential impacts of poultry manure on soil chemical properties and leachates and in particular evaluating the critical application levels (Delgado et al. 2010).
The MS3, from two types of poultry manure (sawdust and straw bed), has also proved to be functional for assessing effects on earthworm, plants and microorganisms on an agricultural land (Delgado et al. 2012), and combined pollutants in contaminated sites (Fernández et al. 2005).
This paper reports the effects on leachates organisms (aquatic invertebrates, algae and in vitro cell line on fish), after applying two types of poultry manure (sawdust or straw bed) on an agricultural land.
Materials and Methods
Multi-species-soil-system test (MS3) is a well-known tool used in order to develop a toxicity test soil and poultry manure (Delgado et al. 2012).
The MS3 was saturated (spring water; 730–940 mL/MS3). After 14 days, no leachates were produced yet and it was decided to keep the irrigation for two more days, until saturation (450 mL, spring water), in order to obtain an acceptable amount of leachates. Chemical properties and ecotoxicity test (Daphnia magna, Chlorella vulgaris and in vitro toxicity on RTG-2 cell) were carried out on the leachates.
Crustacean (D. magna) acute immobilisation test was conducted following the methodology described in ISO guidelines (ISO 1996) with some modifications. For all the samples range finding test were carried out prior to EC50 determinations (OECD 2004). In order to study the acute toxicity test on D. magna the trials were conducted in reconstituted water to Daphnia, for 24 h, after having been kept in agitation with poultry manure. Tests were made at the following dilutions of this extract: 50 %–25 %–12.5 %–6.25 %–3.12 % and 1.56 %. At 48 h there were dead and living individuals. The DL50 (dilution causing 50 % mortality) and its confidence limits 95 % were calculated using the analysis program probit version 1.4. The regression model was verified using Chi square test and T-Student ANOVA to determine the presence of significant differences between the control and treatment groups. These results are incompatible with those obtained by Slomczynska and Slomczynski (2004) using the D. magna test: In this case the period of 24 h was too short to allow the manifestation of the toxic effect.
Algae (C. vulgaris), is a planktonic unicellular green alga growth inhibition test (method described in Ramos et al. 1996). Algae growth was estimated by measuring increases of absorbance (λemission = 450 nm), fluorescence (indicating variations in the chlorophyll content, λemission = 680 nm, λexcitation = 430 nm) and by direct counting using a Neubauer chamber (OECD test guideline 202).
The rainbow trout fish cell line RTG-2 (ATCC CCL55) was grown at 20°C in an incubator and propagated in eagle’s minimum essential medium (EMEM) supplemented with 10 % foetal bovine serum, l-glutamine, non-essential amino acids and penicillin–streptomycin. The cytotoxicity assay was conducted according to Babin and Tarazona (2005). Cell viability was quantified using the neutral red (NR) uptake assay (Borenfreund and Puerner 1985). Presence of co-planar organic toxic chemical was indicated by the stimulation of cytochrome CYP1A, which was measured as 7-ethoxyresorufin-O-deethylase (EROD) activity (Babin and Tarazona 2005). Cellular defence was predicted by hydrolysis of 4-methylumbellferyl-β-D-galactoside (β-gal activity) assay (Babin and Tarazona 2005). The TECAN-Genius spectroflurometer was used to quantify fluorescence (kinetic way) and absorbance endpoints.
Statistical differences for chemical properties of leachates, between poultry-manure amended soil treatments and the control, were assessed by analysing the variance (p ≤ 0.05 and p ≤ 0.01), one-way ANOVA test and the least significant difference (LSD) multiple range test at p ≤ 0.05 and p ≤ 0.01 (SAS Institute 2001).
Results and Discussion
Table 1 reflects the analytical characterization of leachates (volume, pH, electrical conductivity and nitrate). It can be observed a high nitrate concentration in the leachates from the sawdust and straw poultry manure, at time 0 day and 12 days.
On D. magna was high mortality for zero time and almost 100 % and 70 %–80 % mortality for sawdust and straw poultry manure respectively. It can be due the high nitrate concentration in the leachates from both poultries manure.
Figures 1 and 2 show the graphics of the adjusted models (confidence limits 95 %), for the variables: ‘effects’ versus ‘the percentages of dilution for sawdust and straw poultry manure’ respectively.
The percentiles of the adjusted models indicate the value of dilution, in percentage, at which the model reaches certain values. The DL50 had percentile 15.77 (13.43–18.85 95 % CI) for sawdust poultry and percentile 11.06 (9.61–13.13 95 % CI) for straw poultry manure.
The effects on C. vulgaris and in vitro toxicity on RTG-2 cells exposed to leachate were:
No effects, on the inhibition growth of C. vulgaris, were observed after the exposure period (72 h) at any leachates from two different poultry manure. Similar effect was detected by Navas et al. (2006) in the water soluble fraction of the fuel oil.
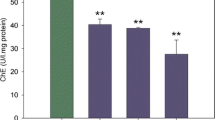
The cytotoxicity assessment of leachates did not reveal toxicity on the poultry manure amended soil. Neutral Red and protein level estimations, as indicators of cytotoxicity indexes, were similar in all treatments (Figs. 3, 4 respectively).
None of the parameters considered; β-gal activity (Fig. 5) and EROD activity (Fig. 6), showed differences between the control and the leachate collected after exposure to poultry manure.
Finally, the capacity of the in vitro assays on fish cell lines, as a complementary tool for analysing effluents, wastes and hot spots has been previously demonstrated (Vega et al. 1996) but limitations should also be considered.
References
Babin MM, Tarazona JV (2005) In vitro toxicity of selected pesticides on RTG-2 and RTL-W1 fish cell lines. Environ Pollut 135:267–274
Borenfreund E, Puerner JÁ (1985) Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett 24:119–124
Delgado M, Martín JV, Miralles de Imperial R, León C, Garcia MC (2010) Phytotoxicity of uncomposted and composted poultry manure. Afr J Plant Sci 4(5):154–162
Delgado M, Rodriguez C, Martín JV, Miralles de Imperial R, Alonso F (2012) Environmental assay on the effect of poultry manure application on soil organisms in agro-ecosystem. Sci Total Environ 416:532–535
Dikinya O, Mufwanzala N (2010) Chicken manure-enhanced soil fertility and productivity: effects of application rates. Soil Sci Environ Manag 1(3):46–54
Fernández C, Cagigal E, Vega M, Tarazona JV (2005) Ecological risk assessment of contaminated soils through direct toxicity assessment. Ecotoxicol Environ Saf 62:174–184
Institute SAS (2001) SAS user’s guide: statistic. SAS inst, Cary, NC
ISO 6341 (1996) Water quality. Determination of the inhibition of the mobility of Daphnia magna straus (Cladocer, Crustacea). Acute toxicity test. 3rd edn
López-Masquera ME, Cabaleiro F, Sainz MS, López-Fabal A, Carral E (2008) Fertilizing value of broiler litter: effects of dryng and pelletizing. Bioresour Technol 99:5626–5633
Navas J, Babin M, Casado S, Fernandez C, Tarazona JV (2006) The Prestige oil spill: a laboratory study about the toxicity of the water-soluble faction of fuel oil. Marine Environ Res 62:s352–s355
OECD (organization for economic cooperation and development) (2004) OCDE guidelines for testing of chemicals. Daphnia sp acute toxicity test. OECD Guideline No 202, Paris, France 2004
Ramos C, de la Torre AI, Tarazona JV, Muñoz MJ (1996) Desarrollo de un ensayo de inhibición del crecimiento de Chlorella vulgaris utilizando un test en microplacas. Rev Toxicol 13:97–100
Slomczynska B, Slomczynski T (2004) Physico-chemical and toxicological characteristics of leachates from MSW landfills. Pol J Environ Stud 6:627–637
Vega M, Fernandez C, Blazquez T, Tarazona JV, Castaño A (1996) Biological and chemical tools in the toxicological risk assessment of Jarama river, Madrid Spain. Environ Pollut 93:135–139
Acknowledgments
We are grateful to INIA-FEDER for funding this study as part of the RTA2005-00120-CO2-01 and RTA2009-00074-00-00 projects. The authors wish to extend their sincere thanks to Ecotoxicology Laboratory for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Delgado, M., de Imperial, R.M., Alonso, F. et al. Ecotoxicity Bioassays on Leachates from Poultry Manure. Bull Environ Contam Toxicol 90, 401–404 (2013). https://doi.org/10.1007/s00128-012-0936-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-012-0936-9