Abstract
Aims/hypothesis
Interventions that reduce inflammation may delay progression of microvascular and macrovascular complications in diabetes. We examined the effects of vitamin D3 and/or n-3 fatty acid supplementation vs placebo on 5 year changes in serum inflammatory and cardiac biomarkers in adults with type 2 diabetes.
Methods
This study reports pre-specified secondary outcomes of the Vitamin D and Omega-3 Trial to Prevent and Treat Diabetic Kidney Disease, in which 1312 US adults with type 2 diabetes and without known cardiovascular disease, malignancy, or end-stage kidney disease were randomised using computer-generated random numbers in blocks of eight to vitamin D3 (2000 IU/day) vs placebo and n-3 fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]; 1 g/day) vs placebo in a 2 × 2 factorial design. Participants, examiners, and researchers assessing outcomes were blinded to intervention assignment. We measured serum IL-6, high-sensitivity C-reactive protein (hsCRP) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) at baseline and after 2 and 5 years.
Results
A total of 333 participants were randomised to vitamin D3 and placebo n-3 fatty acids, 289 to n-3 fatty acids and placebo vitamin D3, 370 to vitamin D3 and n-3 fatty acids, and 320 to 2 placebos; 989 (75%) and 934 (71%) participants returned blood samples at 2 and 5 years, respectively. Participants had a mean age of 67.6 years (46% women). Overall, baseline geometric means of IL-6, hsCRP and NT-proBNP were 1.2 pg/ml, 1.9 mg/l and 262 ng/l, respectively. After 5 years, mean IL-6 and hsCRP remained within 6% of their baseline values while mean NT-proBNP increased by 55% overall. Compared with placebo, participants assigned to vitamin D3 had a 1.24-fold greater increase in NT-proBNP over 5 years (95% CI 1.09, 1.41; p = 0.003), while IL-6 and hsCRP did not have a significant difference in change. Comparing n-3 fatty acids with placebo, there was no significant difference in change in IL-6, hsCRP or NT-proBNP. No heterogeneity was observed in subgroup analyses accounting for baseline eGFR, urine albumin to creatinine ratio, initial biomarker concentration, 25-hydroxyvitamin D level or EPA+DHA index.
Conclusions/interpretation
Among adults with type 2 diabetes, supplementation with vitamin D3 or n-3 fatty acids did not reduce IL-6, hsCRP or NT-proBNP over 5 years.
Trial registration
ClinicalTrials.gov NCT01684722
Funding
The study was funded by grant R01DK088762 from the National Institute of Diabetes and Digestive and Kidney Diseases.

Graphical abstract
Similar content being viewed by others

Introduction
Inflammation has been increasingly implicated in type 2 diabetes and its complications. Immune system activation has been observed in type 2 diabetes and is thought to contribute to the development of atherosclerotic disease, heart failure, kidney disease, retinopathy, neuropathy and impaired wound healing through various mechanisms including leucocyte infiltration, cytokine upregulation and endothelial dysfunction [1, 2]. These processes lead to activation of apoptotic, thrombotic and fibrotic pathways which result in microvascular disruption and tissue damage. Vitamin D compounds and n-3 fatty acids have been demonstrated to reduce inflammation in animal models and human experimental studies, and as such may provide benefit in the management of type 2 diabetes.
While primarily recognised for its role in bone mineral metabolism, 1,25-dihydroxyvitamin D [1,25(OH)2D] also may have important immunomodulatory functions. Immune cells are able to directly synthesise 1,25(OH)2D as well as alter signalling pathways in response to this hormone [3, 4]. Laboratory studies have demonstrated that by regulating gene transcription, 1,25(OH)2D affects the proliferation and differentiation of dendritic cells, macrophages and lymphocytes [5]. These processes have been suggested to result in decreased production of proinflammatory cytokines and immune cell reactivity, though overall results have been inconsistent. In line with these findings, administration of vitamin D receptor agonists has been found to reduce production of inflammatory markers in isolated human monocytes and rat podocytes [6, 7]. Additionally, some but not all epidemiologic studies have demonstrated an inverse association between serum 25-hydroxyvitamin D [25(OH)D] concentrations and diabetes complications, and administration of vitamin D compounds in adults with type 2 diabetes has been observed to reduce markers of endothelial dysfunction and cardiovascular risk, including N-terminal pro-B-type natriuretic peptide (NT-proBNP), in some short-term studies [8,9,10]. However, results have been inconsistent and limited to short-term treatment.
n-3 fatty acids influence immune cell function by altering cell membrane properties, serving as substrates for anti-inflammatory lipid mediators and affecting gene transcription [11]. These mechanisms have primarily been explored in experimental studies in which n-3 fatty acids have been reported to reduce concentrations of inflammatory cytokines, endothelial cell and platelet adhesion, and leucocyte responsiveness [12]. A potential beneficial impact of n-3 fatty acid supplementation on insulin resistance and diabetes complications, including hyperlipidemia, atherosclerotic disease, ventricular remodelling and kidney disease, has also been recognised [13, 14]. Published clinical trials evaluating these effects have been limited, with mixed results [15].
To address whether the previous association study findings underly causal relationships, we examined the effects of vitamin D3 and/or n-3 fatty acid supplementation vs placebo on 5 year changes in serum inflammatory markers (IL-6 and high-sensitivity C-reactive protein [hsCRP]) in a large, randomised, controlled trial of adults with type 2 diabetes. Additionally, we compared concentrations of serum NT-proBNP as a measure of cardiac stress following these interventions. We hypothesised that administration of vitamin D3 and n-3 fatty acids would reduce concentrations of these circulating biomarkers.
Methods
Study design
The Vitamin D and Omega-3 Trial to Prevent and Treat Diabetic Kidney Disease (VITAL-DKD) was designed as an ancillary study to the Vitamin D and Omega-3 Trial (VITAL), which randomised 25,871 participants to vitamin D3 and/or n-3 fatty acids using a placebo-controlled, double-blind, 2 × 2 factorial design and followed them for a median of 5.3 years (ClinicalTrials.gov registration no. NCT01169259) [16]. VITAL-DKD is composed of a subset of participants from the parent VITAL trial with type 2 diabetes for whom longitudinal clinical and laboratory data were collected with the objective of gaining insight into the effects of these interventions on diabetes complications (ClinicalTrials.gov registration no. NCT01684722) [17].
The primary outcome of the VITAL-DKD trial was change in eGFR, for which results were null [18]. This study reports results of pre-specified secondary outcomes of the VITAL-DKD trial: changes in serum concentrations of inflammatory and cardiac markers (IL-6, hsCRP and NT-proBNP) at 2 and 5 years of follow-up.
The study was approved by the institutional review board of Partners HealthCare-Brigham and Women’s Hospital. All participants provided written, informed consent before study enrolment.
Study population
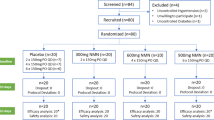
The VITAL trial enrolled men ≥50 years old and women ≥55 years old throughout the USA. Participants with known cardiovascular disease or malignancy (except non-melanoma skin cancer) were excluded from the study, as well as individuals with end-stage kidney disease or on dialysis and individuals at elevated risk for adverse effects from vitamin D supplementation, including those with hypercalcaemia, parathyroid abnormalities, severe liver disease or granulomatous disease [16]. Among these, participants with self-reported physician diagnosis of type 2 diabetes at screening were identified during the VITAL trial placebo run-in period for enrolment in the VITAL-DKD study. Of the 3244 individuals initially invited to take part in the study, ultimately 1312 were included [17, 18]. Those with a diagnosis of diabetes present only during pregnancy (presumed gestational diabetes) or a diagnosis of diabetes made prior to age 30 and requiring treatment with insulin (presumed type 1 diabetes), as well as those with established non-diabetic kidney disease or who had received a kidney transplant, were excluded.
Intervention
Participants in the VITAL study were randomised to one of four treatment groups: (1) vitamin D3 and placebo n-3 fatty acids; (2) n-3 fatty acids and placebo vitamin D3; (3) vitamin D3 and n-3 fatty acids; or (4) both placebos. Participants were asked to limit vitamin D and calcium intake to ≤800 IU and ≤1200 mg daily, respectively, and to avoid n-3 fatty acid supplementation outside of the study protocol. Study investigators and participants were blinded to treatment assignments.
Vitamin D3 2000 IU daily and matching placebo were donated by Pharmavite LLC (USA). n-3 fatty acid 1 g daily (Omacor, 465 mg eicosapentaenoic acid [EPA]+375 mg docosahexaenoic acid [DHA]) and matching placebo were donated by Pronova BioPharma/BASF (USA). All medications were dispensed to participants by mail in monthly calendar packs.
Outcomes
Blood and urine samples were obtained prior to randomisation (hereafter referred to as ‘baseline’), and at 2 and 5 years after randomisation. Samples were collected locally by participants and mailed with frozen gel packs overnight to the central laboratory at the Brigham Women’s Hospital Division of Preventive Medicine. Blood samples were centrifuged then stored at <−80°C as serum aliquots prior to shipment to the University of Washington for measurement of inflammatory markers and NT-proBNP.
A pilot study was performed to confirm the integrity of serum analytes within this sample collection. In the pilot, biomarker concentrations from samples processed immediately correlated strongly (r > 0.99) with those processed after being stored with a frozen gel pack for 24 h prior to processing, with no evidence of bias (electronic supplementary material [ESM] Fig. 1).
hsCRP was measured with a Beckman Coulter (USA) DxC chemistry analyser. Serum IL-6 and NT-proBNP measurements were performed using commercial multiplex electrochemiluminescence assays (Meso Scale Discovery, Rockville, MD, USA). The lower limits of detection for measures of IL-6, hsCRP and NT-proBNP were 0.06 pg/ml, 0.2 mg/l and 0.58 ng/l, respectively. Samples below detection limits were found for hsCRP and these were assigned the value 0.2 mg/l. Values of measurements for blind replicates introduced into the assay platform on different dates were highly correlated, using the Pearson method: IL-6, r = 0.91; hsCRP, r > 0.99; NT-proBNP, r > 0.99. To avoid laboratory drift, all reagents, kits, calibrators and controls were obtained from the same lot.
Clinical characteristics and covariates
Questionnaire data from the parent VITAL trial obtained at baseline and yearly thereafter provided information on participant demographics, changes in health status, use of non-study vitamin D and n-3 fatty acid supplements, dietary vitamin D and fish intake, and adherence to study medications. High adherence over 2 and 5 years was defined as self-reported adherence to at least two-thirds of study medications on at least two out of three questionnaires over 2 years, and on at least four out of six questionnaires over 5 years, respectively (with questionnaires administered at 6 months and 1, 2, 3, 4 and 5 years after randomisation). Additional VITAL-DKD questionnaire data were obtained at baseline with a focus on diabetes-related issues, namely use of anti-hyperglycaemic and antihypertensive agents and the presence of diabetes complications.
Serum 25(OH)D measurements were obtained using LC-MS/MS [19]. Plasma n-3 fatty acids were assayed by LC-MS/MS and quantified as combined EPA and DHA indices indicating percentage of total fatty acids [20]. Measurements of creatinine and cystatin C were performed with a Beckman Coulter DxC chemistry analyser.
Statistical analyses
Linear mixed models tested the effects of each intervention independently on changes in log-transformed concentrations of inflammatory markers and NT-proBNP. Time (modelled as indicator variables for years 2 and 5), treatment and interactions of treatment with time were included as independent variables. A single Wald test of the coefficients for the interaction of treatment with both time indicators was used as the measure of treatment effect for each outcome. We assessed three outcomes for each intervention and used a Bonferroni correction to adjust for multiple corrections, thus considering a p value of less than 0.05/3 = 0.0167 as statistically significant. Multiple imputation using chained equations was employed to account for missing data, including missing outcomes, with resulting estimates combined using Rubin’s rules [21]. Analyses were adjusted for pre-specified baseline characteristics including age, sex, baseline eGFR and baseline urine albumin to creatinine ratio (UACR) by adding covariates and interactions of covariates with time into each linear mixed model.
We performed exploratory sensitivity analyses to consider participants’ self-reported adherence to each treatment. Additionally, for each intervention we explored effect modification for changes in biomarker concentrations from baseline to year 5 by the following pre-specified covariates: race, baseline BMI, UACR, eGFR and biomarker concentrations. In the vitamin D3 arm we examined the treatment effect of baseline total serum 25(OH)D concentration stratified by clinical cutoffs for vitamin D sufficiency. In the n-3 fatty acid arm we examined the treatment effects of baseline EPA+DHA indices and dietary fish intake stratified by their overall median values. Effect modification was assessed by adding an indicator of the covariate of interest stratum as well as its interaction with time to the linear mixed models. We used linear combinations of resulting estimates to report changes in study outcomes according to covariate subgroup and treatment assignment.
All analyses were done using the R 3.6.0 statistical computing environment (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participant characteristics
A total of 1312 individuals were included in the study (see ESM Fig. 2 for CONSORT Flow Diagram). At baseline, the mean age of participants was 67.6 years; 46% of participants were women, and 22% identified as black (Table 1). Over half of participants had had diabetes for greater than 5 years, with the median reporting a diabetes duration of 6–10 years. The mean BMI was 31.4 kg/m2. Among this cohort, biguanides were the most commonly reported glucose-lowering medications used (in 68%), followed by sulfonylurea agents (in 30%) and insulin (in 20%). The majority of participants were prescribed antihypertensive medications (predominantly ACE inhibitors [ACEis] or angiotensin II receptor blockers [ARBs]) and cholesterol-lowering agents. Most participants had normal eGFR at baseline, with a mean eGFR of 85.8 ml min−1 1.73 m−2 and median UACR of 0.34 mg/mmol. Fifty-four per cent of participants had a baseline 25(OH)D level of <75 nmol/l. At randomisation, 41% reported supplemental vitamin D use of ≤800 IU/day. Demographic and clinical factors were well balanced across treatment arms.
Participant retention and adherence
At least one follow-up blood sample was provided by 83% of participants (n = 1090) over the study duration. Baseline demographic and clinical variables were generally similar when comparing participants who did not return a follow-up blood sample with those who did (ESM Table 1). Seventy-five per cent (n = 989) and 71% of participants (n = 934) provided samples at 2 and 5 years, respectively [18]. Among all participants randomised into the trial, participant adherence to vitamin D3 treatment randomisation was 91% and 79% over 2 and 5 years, respectively, while adherence to n-3 treatment randomisation was 91% and 79%.
Significant differences in mean serum 25(OH)D and EPA+DHA indices were observed at 2 years in intervention groups compared with placebo (p < 0.001). Participants assigned to vitamin D3 achieved a mean serum 25(OH)D concentration of 103.4 nmol/l compared with 74.4 nmol/l in those receiving placebo, and participants assigned to n-3 achieved a mean plasma EPA+DHA index of 3.6% compared with 2.3% in those receiving placebo [18].
There were no changes in measures of kidney function including eGFR and UACR by treatment assignment at 2 or 5 years of follow-up [18]. Biguanide, sulfonylurea, insulin and ACEi use among participants was largely stable over the course of the study. Over 5 years, use of dipeptidyl peptidase-4 inhibitors and ARBs increased by 5% and 9%, respectively. Five per cent of participants were started on a sodium–glucose cotransporter 2 inhibitor. These changes were similar across intervention groups.
Change in biomarker concentrations
Overall, concentrations of IL-6 and hsCRP remained stable throughout the follow-up period while NT-proBNP increased over time (Fig. 1). In the full population, baseline geometric means of IL-6, hsCRP and NT-proBNP were 1.21 pg/ml (SD, 3.02), 1.94 mg/l (SD, 3.13) and 262 ng/l (SD, 3.7), respectively. Whereas IL-6 and hsCRP remained within 6% of their initial concentrations after 5 years of follow-up (ESM Table 2), concentrations of NT-proBNP rose by 55% (geometric mean at 5 years 406.6 ng/l, SD 4.1).
Comparing vitamin D3 with placebo, there were no significant differences in change of IL-6 or hsCRP concentrations over time (Table 2). Ratios of change at 5 years were 0.96 (95% CI 0.79, 1.16) for IL-6 and 0.90 (95% CI 0.79, 1.03) for hsCRP. NT-proBNP increased by 72% over 5 years among those receiving vitamin D3 vs 38% among those receiving placebo. This represents a 1.24-fold greater increase in NT-proBNP concentration over time in response to vitamin D3 (95% CI 1.09, 1.41; global test for ratio of change p = 0.003). Comparing n-3 fatty acids with placebo, there were no significant differences in change of IL-6, hsCRP or NT-proBNP concentrations over time after accounting for multiple comparisons (Table 3). Ratios of change at 5 years were 1.08 (95% CI 0.88, 1.31) for IL-6, 0.86 (95% CI 0.76, 0.98) for hsCRP and 1.01 (95% CI 0.88, 1.16) for NT-proBNP.
Sensitivity analyses
Results among highly adherent participants mirrored those obtained in the complete cohort analyses. In this group, the ratio of change in NT-proBNP concentration among those receiving vitamin D3 compared with placebo was 1.24 at 5 years (95% CI 1.09, 1.43; global test for ratio of change p = 0.007; ESM Table 3). No significant differences in change in biomarker concentrations were observed with high adherence to n-3 compared with placebo (ESM Table 4).
Differences in change in biomarker concentrations from baseline to 5 years were compared across pre-specified subgroups. No significant heterogeneity was observed for biomarkers with either of the assigned interventions (ESM Tables 5–10, ESM Figs 3–8). Furthermore, no heterogeneity was observed by baseline vitamin D status when considering concentrations <30 nmol/l, though these sample sizes were small (data not included).
Discussion
Among adults with type 2 diabetes, daily supplementation with vitamin D3 or n-3 fatty acids did not reduce serum concentrations of IL-6, hsCRP or NT-proBNP over 5 years of follow-up. Concentrations of IL-6 and hsCRP remained steady while NT-proBNP increased over time, with greater increases among those randomised to vitamin D3 compared with placebo. Results remained consistent in adherence-based sensitivity analyses. There was no difference in biomarker concentration in response to supplementation in subgroup analyses.
Our findings are consistent with the parent VITAL trial, which did not demonstrate a reduction in major cardiovascular events or invasive cancer with either intervention, though treatment with vitamin D showed a signal for reduction in cancer death and n-3 fatty acids were associated with decreased incidence of myocardial infarction, evaluated as a secondary outcome [19, 20]. Within this same population, VITAL-HF (Vitamin D and Omega-3 Trial—Heart Failure) found no effect of vitamin D3 and n-3 fatty acids on incidence of first heart failure hospitalisation, though daily n-3 fatty acids had a beneficial effect on recurrent heart failure hospitalisation [22]. VITAL-DKD found no effect of either intervention on change in eGFR or urine albumin excretion [18].
In the context of null results for most clinical outcomes to date, examining effects of intermediate mechanistic outcomes serves several important purposes. First, a decrease in inflammatory markers would suggest potential value for these supplements on diabetes outcomes not assessed in the studies above, such as retinopathy or neuropathy. Second, changes in biomarkers could portend a delayed benefit on cardiovascular or kidney outcomes that were not observed during the aforementioned trials. However, in this study we did not observe any reductions in three key hypothesised mediators in response to either of the VITAL interventions. Consistent with our findings, another recently published ancillary study from VITAL evaluating 1 year changes in IL-6, TNF receptor 2 and hsCRP in a subset of 1561 individuals from the general VITAL study cohort who had provided blood samples found that neither vitamin D3 nor n-3 fatty acids reduced these inflammatory markers, noting instead a significant increase in IL-6 concentration with vitamin D3 compared with placebo [23].
Three meta-analyses reported a reduction in hsCRP with use of vitamin D compounds, while treatment effects varied for other inflammatory markers including IL-6, TNF-α and erythrocyte sedimentation rate [24,25,26]. Subgroup analyses did not reveal significant heterogeneity when considering baseline vitamin D status and dose and duration of vitamin D supplementation [25, 26], though they demonstrated greater reductions in hsCRP in the presence of coronary heart disease and end-stage kidney disease on haemodialysis [24], two populations that were excluded in our study. Notably, two of the three meta-analyses reported publication bias for the effect of vitamin D supplementation on hsCRP [24, 26]. Additionally, results of these meta-analyses are limited by short follow-up periods and use of varied formulations, doses and durations of vitamin D therapy across included studies. Studies assessing vitamin D supplementation in other populations have also reported mixed results—a meta-analysis of seven RCTs consisting of participants with chronic heart failure observed significant decrease in C-reactive protein (CRP) and TNF-α [27], while a meta-analysis of 13 RCTs of overweight and obese individuals did not find a reduction in CRP, IL-6 or TNF-α with intervention [28].
An unexpected finding in our study was that those taking vitamin D3 had a 24% greater increase in NT-proBNP compared with placebo. Participants in our study had mean NT-proBNP concentrations that were in the normal range at baseline and rose to the upper-normal range over time, perhaps due to vitamin D3 supplementation. This contradicts animal studies demonstrating reduced B-type natriuretic peptide (BNP) transcription with vitamin D receptor activation [29,30,31] and clinical studies reporting either a neutral or beneficial impact of vitamin D receptor agonists on markers of endothelial and cardiac function [10, 27, 32]. In a large cohort study of patients with systolic heart failure, low 25(OH)D levels were associated with elevated NT-proBNP [33]. One clinical trial randomising 61 participants with type 2 diabetes to a single high dose of oral vitamin D3 vs placebo reported a significant decrease in BNP after 16 weeks [9]. Additionally, the Paricalcitol Capsule Benefits in Renal Failure-Induced Cardiac Morbidity (PRIMO) study found that randomisation to paricalcitol compared with placebo attenuated rise of BNP concentrations in participants with [34]. It also seems unlikely that vitamin D supplementation would result in abnormal cardiac remodelling by means of increased fibroblast growth factor 23 (FGF23) considering that in Chronic Renal Insufficiency Cohort Study (CRIC) participants increased left ventricular mass was most strongly observed in those with high FGF23 in the presence of low 25(OH)D [35]. Our contrasting findings are particularly surprising considering that neither the parent VITAL trial nor VITAL-HF reported a parallel increase in major cardiovascular events or heart failure hospitalisations in the vitamin D supplementation group, though our study sample with diabetes is a subset of those included in these other trials [22]. We were unable to find evidence from basic science or clinical research literature to explain our results, though regression to the mean could partially account for our findings as the vitamin D3 group had lower concentrations of NT-proBNP at baseline compared with the placebo group.
Large clinical trials of n-3 fatty acids have reported a reduction in cardiovascular events and mortality as well as improvement in left ventricular structure and function in heart failure populations [36,37,38]. Many smaller trials have focused on the effects of n-3 fatty acids on inflammatory markers. A meta-analysis of 68 RCTs reported significant decreases in CRP, TNF-α and IL-6 in response to a variety of n-3 fatty acid preparations and doses [39]. In meta-regression analysis, each g/day higher dose of EPA was associated with a 17.7% reduction in geometric mean of CRP. However, only seven of the included studies focused on a diabetes population, and these reported inconsistent effects of n-3 fatty acids on inflammatory markers. We found that 5 year hsCRP concentrations were lower in the group receiving n-3 fatty acid compared with placebo, though this association was not significant when accounting for multiple testing. Notably, the dose of n-3 fatty acids used in our study is small compared with the majority of those in the meta-analysis, with most included studies using >1 g of total EPA+DHA. In addition, a newer formulation of purified n-3 acids was recently found to be superior vs placebo in reducing cardiovascular events [40]. Higher doses and/or higher proportions of EPA within n-3 fatty acid formulations may have more success in reducing inflammatory markers and NT-proBNP compared with that studied in VITAL.
Strengths of our study include its randomised placebo-controlled design, large sample size, long follow-up period and strong participant adherence. Our study also has several limitations. Only a limited number of inflammatory and cardiac markers were selected for study. It is possible that our interventions had effects on immune activity and cardiac function that were not reflected in these selected serum biomarkers. Also, biomarker concentrations could have been affected by unaccounted participant-specific events such as changes in dietary practices or acute illness, though these are unlikely to have differed by treatment assignment. Additionally, our study included relatively few participants with baseline serum 25(OH)D concentrations <30 nmol/l, among whom vitamin D supplementation may plausibly have a larger effect [41, 42], though we did not observe heterogeneity in treatment effect by baseline 25(OH)D concentration.
In conclusion, supplementation with 2000 IU daily of vitamin D3 or 1 g daily of n-3 fatty acids in adults with type 2 diabetes did not reduce concentrations of inflammatory biomarkers or NT-proBNP. Contrary to our expectations, we observed a significant increase in NT-proBNP with vitamin D3 compared with placebo. Our study contributes to a growing body of literature that suggests the observed benefits of vitamin D3 and n-3 fatty acids in laboratory studies may not directly translate to clinical benefit.
Data availability
De-identified individual participant data and a data dictionary defining each field in the set will be made available to others beginning 8 November 2021. Data will be made available for reproduction of trial results after approval of a proposal, without investigator support. To access the data, please contact climonte@uw.edu.
Change history
10 December 2020
A Correction to this paper has been published: <ExternalRef><RefSource>https://doi.org/10.1007/s00125-020-05339-6</RefSource><RefTarget Address="10.1007/s00125-020-05339-6" TargetType="DOI"/></ExternalRef>
Abbreviations
- ACEi:
-
ACE inhibitor
- ARB:
-
Angiotensin II receptor blocker
- BNP:
-
B-type natriuretic peptide
- CRP:
-
C-reactive protein
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- FGF23:
-
Fibroblast growth factor 23
- hsCRP:
-
High-sensitivity C-reactive protein
- NT-proBNP:
-
N-terminal pro-B-type natriuretic peptide
- 1,25(OH)2D:
-
Dihydroxyvitamin D
- 25(OH)D:
-
25-hydroxyvitamin D
- UACR:
-
Urine albumin to creatinine ratio
- VITAL:
-
Vitamin D and Omega-3 Trial
- VITAL-DKD:
-
Vitamin D and Omega-3 Trial to Prevent and Treat Diabetic Kidney Disease
- VITAL-HF:
-
Vitamin D and Omega-3 Trial—Heart Failure
References
Jha JC, Ho F, Dan C, Jandeleit-Dahm K (2018) A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin Sci 132(16):1811–1836. https://doi.org/10.1042/CS20171459
Pichler R, Afkarian M, Dieter BP, Tuttle KR (2017) Immunity and inflammation in diabetic kidney disease: translating mechanisms to biomarkers and treatment targets. Am J Physiol Renal Physiol 312(4):F716–F731. https://doi.org/10.1152/ajprenal.00314.201
Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M (2003) Regulation of 25-hydroxyvitamin D3-1α-hydroxylase and production of 1α,25-dihydroxyvitamin D3 by human dendritic cells. Blood 102(9):3314–3316. https://doi.org/10.1182/blood-2002-11-3521
Kreutz M, Andreesen R, Krause SW, Szabo A, Ritz E, Reichel H (1993) 1,25-Dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood 82(4):1300–1307. https://doi.org/10.1182/blood.v82.4.1300.bloodjournal8241300
Colotta F, Jansson B, Bonelli F (2017) Modulation of inflammatory and immune responses by vitamin D. J Autoimmun 85:78–97. https://doi.org/10.1016/j.jaut.2017.07.007
Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C (2007) Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D3 works as anti-inflammatory. Diabetes Res Clin Pract 77(1):47–57. https://doi.org/10.1016/j.diabres.2006.10.007
Sanchez-Niño MD, Bozic M, Córdoba-Lanús E et al (2012) Beyond proteinuria: VDR activation reduces renal inflammation in experimental diabetic nephropathy. Am J Physiol Renal Physiol 302(6):647–657. https://doi.org/10.1152/ajprenal.00090.2011
Sacerdote A, Dave P, Lokshin V, Bahtiyar G (2019) Type 2 diabetes mellitus, insulin resistance, and vitamin D. Curr Diab Rep 19(10):101. https://doi.org/10.1007/s11892-019-1201-y
Witham MD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD (2010) The effect of different doses of vitamin D3 on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia 53(10):2112–2119. https://doi.org/10.1007/s00125-010-1838-1
Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD (2008) Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabet Med 25(3):320–325. https://doi.org/10.1111/j.1464-5491.2007.02360.x
Calder PC (2010) Omega-3 fatty acids and inflammatory processes. Nutrients 2(3):355–374. https://doi.org/10.3390/nu2030355
De Caterina R, Madonna R, Bertolotto A, Schmidt EB (2007) n-3 fatty acids in the treatment of diabetic patients: biological rationale and clinical data. Diabetes Care 30(4):1012–1026. https://doi.org/10.2337/dc06-1332
Tessaro FHG, Ayala TS, Martins JO (2015) Lipid mediators are critical in resolving inflammation: a review of the emerging roles of eicosanoids in diabetes mellitus. Biomed Res Int 2015:568408. https://doi.org/10.1155/2015/568408
Poudyal H, Brown L (2013) The role of n-3 polyunsaturated fatty acids in human heart failure. Endocr Metab Immune Disord Drug Targets 13(1):105–117. https://doi.org/10.2174/1871530311313010013
Wong CY, Yiu KH, Li SW et al (2010) Fish-oil supplement has neutral effects on vascular and metabolic function but improves renal function in patients with type 2 diabetes mellitus. Diabet Med 27(1):54–60. https://doi.org/10.1111/j.1464-5491.2009.02869.x
Manson JE, Bassuk SS, Lee I et al (2012) The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials 33(1):159–171. https://doi.org/10.1016/j.cct.2011.09.009
de Boer IH, Zelnick LR, Lin J et al (2018) Vitamin D and omega-3 trial to prevent and treat diabetic kidney disease: rationale, design, and baseline characteristics (VITAL-DKD). Contemp Clin Trials 74:11–17. https://doi.org/10.1016/j.cct.2018.09.014
de Boer IH, Zelnick LR, Ruzinski J et al (2019) Effect of vitamin D and omega-3 fatty acid supplementation on kidney function in patients with type 2 diabetes: a randomized clinical trial. JAMA 322(19):1899–1909. https://doi.org/10.1001/jama.2019.17380
Manson JE, Cook NR, Lee I-M et al (2019) Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 380(1):33–44. https://doi.org/10.1056/NEJMoa1809944
Manson JAE, Cook NR, Lee IM et al (2019) Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 380(1):23–32. https://doi.org/10.1056/NEJMoa1811403
Rubin D (1987) Multiple imputation for nonresponse in surveys. Wiley, New York
Djoussé L, Cook NR, Kim E, Manson JE, Buring JE, Albert CM (2019) Supplementation with vitamin D and/or omega-3 fatty acids and incidence of heart failure hospitalization: VITAL-Heart Failure. Circulation 141:784–786. https://doi.org/10.1161/CIRCULATIONAHA.119.044645.23
Costenbader KH, Macfarlane LA, Lee I et al (2019) Effects of one year of vitamin D and marine omega-3 fatty acid supplementation on biomarkers of systemic inflammation in older US adults. Clin Chem 65(12):1508–1521. 000. https://doi.org/10.1373/clinchem.2019.306902
Mansournia M, Ostadmohammadi V, Doosti-Irani A et al (2018) The effects of vitamin D supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res 50(6):429–440. https://doi.org/10.1016/j.pnpbp.2019.109651.
Mousa A, Naderpoor N, Teede H, Scragg R, de Courten B (2018) Vitamin D supplementation for improvement of chronic low-grade inflammation in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev 76(5):380–394. https://doi.org/10.1093/nutrit/nux077
Yu Y, Tian L, Xiao Y, Huang G, Zhang M (2018) Effect of vitamin D supplementation on some inflammatory biomarkers in type 2 diabetes mellitus subjects: a systematic review and meta-analysis of randomized controlled trials. Ann Nutr Metab 73(1):62–73. https://doi.org/10.1159/000490358
Jiang WL, Gu HB, Zhang YF, Xia QQ, Qi J, Chen JC (2016) Vitamin D supplementation in the treatment of chronic heart failure: a meta-analysis of randomized controlled trials. Clin Cardiol 39(1):56–61. https://doi.org/10.1002/clc.22473
Jamka M, Woźniewicz M, Jeszka J, Mardas M, Bogdański P, Stelmach-Mardas M (2015) The effect of Vitamin D supplementation on insulin and glucose metabolism in overweight and obese individuals: systematic review with meta-analysis. Sci Rep 5:114–116. https://doi.org/10.1038/srep16142
Chen S, Glenn DJ, Ni W et al (2008) Expression of the vitamin D receptor is increased in the hypertrophic heart. Hypertension. 52(6):1106–1112. https://doi.org/10.1161/HYPERTENSIONAHA.108.119602
Choudhury S, Bae S, Ke Q et al (2014) Abnormal calcium handling and exaggerated cardiac dysfunction in mice with defective vitamin D signaling. PLoS One 9(9):2–10. https://doi.org/10.1371/journal.pone.0108382
Kong J, Kim GH, Wei M et al (2010) Therapeutic effects of vitamin D analogs on cardiac hypertrophy in spontaneously hypertensive rats. Am J Pathol 177(2):622–631. https://doi.org/10.2353/ajpath.2010.091292
Tabrizi R, Akbari M, Lankarani KB, Heydari ST, Kolahdooz F, Asemi Z (2018) The effects of vitamin D supplementation on endothelial activation among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab (Lond) 15(1):85. https://doi.org/10.1186/s12986-018-0320-9
Liu LCY, Voors AA, Van Veldhuisen DJ et al (2011) Vitamin D status and outcomes in heart failure patients. Eur J Heart Fail 13(6):619–625. https://doi.org/10.1093/eurjhf/hfr032
Thadhani R, Appelbaum E, Pritchett Y et al (2012) Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA 307(7):674–684. https://doi.org/10.1001/jama.2012.120
Ky B, Shults J, Keane MG et al (2013) FGF23 modifies the relationship between vitamin D and cardiac remodeling. Circ Heart Fail 6(4):817–824. https://doi.org/10.1161/CIRCHEARTFAILURE.112.000105
Tavazzi L, Maggioni AP, Marchioli R et al (2008) Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 372(9645):1231–1239. https://doi.org/10.1016/S0140-6736(08)61239-8
Yokoyama M, Origasa H, Matsuzaki M et al (2007) Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369(9567):1090–1098. https://doi.org/10.1016/S0140-6736(07)60527-3
Siscovick DS, Barringer TA, Fretts AM et al (2017) Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a science advisory from the American Heart Association. Circulation 135(15):e867–e884. https://doi.org/10.1161/CIR.0000000000000482
Li K, Huang T, Zheng J, Wu K, Li D (2014) Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor α: a meta-analysis. PLoS One 9(2):1–28. https://doi.org/10.1371/journal.pone.0088103
Bhatt DL, Steg PG, Miller M et al (2019) Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 380(1):11–22. https://doi.org/10.1056/NEJMoa1812792
Zhang R, Li B, Gao X et al (2017) Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose-response meta-analysis of prospective studies. Am J Clin Nutr 105(4):810–819. https://doi.org/10.3945/ajcn.116.140392
Crowe FL, Thayakaran R, Gittoes N et al (2019) Non-linear associations of 25-hydroxyvitamin D concentrations with risk of cardiovascular disease and all-cause mortality: results from The Health Improvement Network (THIN) database. J Steroid Biochem Mol Biol 195:105480. https://doi.org/10.1016/j.jsbmb.2019.105480
Acknowledgements
We are indebted to the VITAL participants and to the entire VITAL research group and staff for their dedicated collaboration. Voting members of the VITAL Data and Safety Monitoring Board include L. S. Cohen (Yale University, School of Medicine, USA), T. Colton (Boston University, USA), M. A. Espeland (Wake Forest University, USA), C. Henderson (UCSF, USA), A. H. Lichtenstein (Tufts University, USA), R. A. Silliman (Boston University, USA) and N. K. Wenger (chair Emory University, USA); these members received a National Institutes of Health (NIH)-set stipend for their service. Ex-officio members at the NIH (USA) include J. Boyington, R. B. Costello, C. D. Davis, P. Greenwald, G. Riscuta and H. Seifried.
Authors’ relationships and activities
IHdB has consulted for Ironwood and Boehringer-Ingleheim and received equipment and supplies for research from Medtronic and Abbott. RT has consulted for Fresenius Medical Care North America. JEB’s spouse is on the Scientific Advisory Board of Pharmavite, who provided pills for the trial.
Funding
This study was funded by grant R01DK088762 from the National Institute of Diabetes and Digestive and Kidney Diseases. The parent trial was funded by grants U01CA138962 and R01CA138962, which include support from the National Cancer Institute; the National Heart, Lung, and Blood Institute; the Office of Dietary Supplements; the National Institute of Neurological Disorders and Stroke; and the National Center for Complementary and Integrative Health. Study pills were donated by Pharmavite and Pronova BioPharma/BASF. Additional support was provided by grant T32DK007467 from the National Institute of Diabetes and Digestive and Kidney Diseases, and an unrestricted fund from the Northwest Kidney Centers. The funding organisations had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
CPL conducted the literature search and drafted the manuscript. LRZ conducted the data analysis and created the tables and figures. JR conducted laboratory measurements of biosamples. JEB, HDS and JEM serve as primary investigators of the VITAL cohort and supervised the project. IHdB serves as primary investigator of the VITAL-DKD cohort and supervised the project. All authors contributed to data interpretation, and read, edited and approved the final manuscript. CPL had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The graphical abstract included a typographical error (1000 g n-3 fatty acid supplementation, rather than 1000 mg).
Electronic supplementary material
ESM
(PDF 6325 kb)
Rights and permissions
About this article
Cite this article
Limonte, C.P., Zelnick, L.R., Ruzinski, J. et al. Effects of long-term vitamin D and n-3 fatty acid supplementation on inflammatory and cardiac biomarkers in patients with type 2 diabetes: secondary analyses from a randomised controlled trial. Diabetologia 64, 437–447 (2021). https://doi.org/10.1007/s00125-020-05300-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-020-05300-7