Abstract
Aims/hypothesis
The benefits of moderate alcohol consumption for type 2 diabetes have been postulated to involve a mechanism of improved insulin sensitivity. Fetuin-A, which is known to inhibit insulin signalling, has emerged as a biomarker for diabetes risk. Alcohol consumption may influence circulating fetuin-A concentrations and subsequently diabetes risk by altering the insulin signal. We therefore hypothesised that moderate alcohol consumption would be associated with lower fetuin-A concentration and that fetuin-A would partly explain the association between alcohol consumption and incident type 2 diabetes.
Methods
Among diabetes-free female participants in the Nurses’ Health Study (n = 1,331), multiple linear regression was conducted to assess the association between alcohol consumption and plasma fetuin-A. Least-squares means (lsmeans) of fetuin-A were estimated in categories of alcohol consumption (0, 0.1–4.9, 5–14.9 and ≥15 g/day). The proportion of alcohol consumption and diabetes association explained by baseline fetuin-A was assessed in 470 matched incident diabetes case–control pairs with follow-up 2000–2006.
Results
Higher alcohol consumption was associated with lower plasma fetuin-A (p for trend = 0.009): lsmean ± SE 476.5 ± 5.9 μg/ml for abstainers, 468.9 ± 5.2 μg/ml for 0.1–4.9 g/day consumers, 455.9 ± 7.0 μg/ml for 5.0–14.9 g/day consumers, and 450.0 ± 9.4 μg/ml for ≥15.0 g/day consumers. Fetuin-A and fasting insulin explained 18.4% and 54.8%, respectively, of the inverse association between alcohol consumption and diabetes after multiple adjustment (both p for contribution <0.04).
Conclusions/interpretation
Moderate alcohol consumption is associated with lower plasma fetuin-A in diabetes-free women. Fetuin-A and insulin explain a significant proportion of the association between alcohol consumption and incident type 2 diabetes. Further studies are needed to examine potential biological mechanisms underlying this association.
Similar content being viewed by others
Introduction
Moderate alcohol consumption is known to have beneficial effects on glucose metabolism [1, 2]. Based on a meta-analysis of 20 prospective cohort studies, moderate alcohol intake is consistently associated with a reduced risk of type 2 diabetes [1]. Despite this evidence, alcohol consumption recommendations for diabetes prevention remain controversial because alcohol intake at an excess level may cause pancreatic damage and thus increase risk of type 2 diabetes as well as contribute to liver injuries [1, 3]. In addition, the physiological explanation for the beneficial effect of moderate alcohol consumption on type 2 diabetes is unclear, although it has been suggested that the mechanism may involve improving insulin sensitivity [4–6], potentially regulated by other biological pathways.
Recently, fetuin-A, which is a glycoprotein secreted by the liver, has emerged as a biomarker for risk of type 2 diabetes [7–12]. Fetuin-A is involved in the mechanism regulating the insulin signalling pathway through inhibition of insulin receptor tyrosine kinase activity [13–15]. Dysregulation of this process may result in the pathophysiological sequelae leading to type 2 diabetes [13]. In large cohort studies, elevated plasma fetuin-A was associated with increased risk of incident type 2 diabetes [7–10]. In addition, fetuin-A has been found to correlate inversely with adiponectin [16], which has insulin-sensitising properties [17]. Thus, we speculate that fetuin-A may be involved in the altered insulin signalling and improved insulin sensitivity induced by alcohol consumption.
In other words, alcohol consumption may influence circulating fetuin-A concentrations, and subsequently diabetes risk, by altering the insulin signal. However, no large-scale human studies have investigated this question. We therefore hypothesised that moderate alcohol consumption would be associated with lower plasma fetuin-A concentration in individuals without diabetes, and that plasma fetuin-A would partly explain the association between alcohol consumption and incident type 2 diabetes in a large population of women.
Methods
Study population
The Nurses’ Health Study is a prospective cohort study of 121,700 female registered nurses aged 30–55 years living across the USA at the baseline data collection in 1976. The participants have been followed biennially with questionnaires on medical history and lifestyle. Blood sample collections were conducted on 32,826 participants in 1989–1990 (biospecimen collection cycle 1) and 18,717 participants in 2000–2001 (cycle 2). Among participants who provided a blood sample, a prospective nested case–control approach was used to examine the association of plasma biomarkers in relation to specific disease risk. For the current investigation on the association of alcohol consumption with plasma fetuin-A, we included participants who were previously selected as controls for type 2 diabetes from blood collection cycle 2, coronary heart disease from cycle 1, or stroke from cycle 1 of a nested case–control study. We excluded participants with self-reported prevalent diabetes and cancer at blood draw. A total of 1,331 individuals with data on both alcohol consumption and plasma fetuin-A were included in the analysis of the association between alcohol consumption and fetuin-A in diabetes-free women.
For incident type 2 diabetes analysis, a nested case–control study of diabetes was conducted among women with an available blood sample. We prospectively identified and confirmed 470 patients with type 2 diabetes from the date of blood draw in 2000 through 2006 who were free of self-reported prevalent diabetes, cardiovascular disease and cancer at blood draw [7]. Risk-set sampling was used to randomly select one control for each case from the rest of the population who remained free of diabetes when the case was diagnosed [18]. Participants were further matched cases and controls for age at blood draw (±1 year), date of blood draw (±3 months), fasting status (fast for ≥8 h) and ethnicity (white/non-white). We excluded cases of diabetes diagnosed in the first year since blood collection in order to minimise reverse causation bias. The study protocol was approved by the institutional review board of the Brigham and Women’s Hospital and the Human Subjects Committee Review Board of Harvard School of Public Health. Informed consent was obtained from all participants.
Assessment of diet
Dietary intake has been assessed using a validated semiquantitative Food Frequency Questionnaire (FFQ) every 4 years as described in detail previously [19, 20]. Participants were asked to select their usual intake of a standard portion of each food item. The daily nutrient intake was estimated using the Harvard Food Composition Database derived from the US Department of Agriculture nutrient data. Questions about consumption of wine, beer and spirits (i.e. distilled alcohol) were asked as separate items [21]. Ethanol intake was estimated by multiplying the frequency of consumption of each beverage by the alcohol content of the specified portion size (11.0 g for wine, 12.8 g for beer, and 14.0 g for spirits) and summing across beverages to calculate total alcohol intake. The assessment of alcohol intake by FFQ correlated highly with intake assessed by multiple-week dietary records (Spearman r = 0.90 for total alcohol, 0.83 for wine, 0.81 for beer, and 0.80 for spirits) and was highly reproducible over the 1-year interval (r = 0.90 for total alcohol) [22, 23]. To reduce measurement errors, the cumulative average intake value of two FFQs, one closest to the time of blood collection and another 4 years preceding it (i.e. 1986 and 1990 FFQs for corresponding blood collection cycle 1 and 1994 and 1998 FFQs for cycle 2), were computed for all dietary variables including alcohol.
Assessment of plasma fetuin-A and biochemical analysis
Blood sample collection and treatment have been described in detail previously [7]. Samples were processed immediately upon arrival. Aliquots were placed into cryotubes as plasma, buffy coat and erythrocytes. All cryotubes were stored in the vapour phase of liquid nitrogen freezers at −130°C or less. Plasma fetuin-A concentrations were measured in singlicate by an enzyme immunoassay from R&D Systems (Minneapolis, MN, USA). Based on the quality control samples (n = 80) run with the study samples, the average intra-assay CV for fetuin-A was 13.1%. C-reactive protein (CRP; intra-assay CV 1.2%), HbA1c (intra-assay CV 5.3%), fasting insulin (intra-assay CV 3.3%), adiponectin (intra-assay CV 8.0%), liver alanine aminotransferase (ALT; intra-assay CV 7.9%) and γ-glutamyl transpeptidase (GGT; intra-assay CV 3.4%) were measured as described previously [7]. For diabetes case–control analysis, each pair was shipped in the same batch and analysed in the same run.
Assessment of other covariates
Information on medical history, lifestyle practices and body weight was collected at baseline and has been updated every 2 years. For the present analysis, we included covariates derived from the questionnaires obtained closest to the time of blood collection. BMI was calculated as weight (kg) divided by height squared (m2). Based on the previous validation study, self-reported weights correlate highly with measured weights (r = 0.97) [24]. Information on cigarette smoking, physical activity, family history of diabetes, postmenopausal hormone use, and history of hypertension or hypercholesterolaemia was assessed from these questionnaires. The validity of these assessments has been documented previously [25].
Ascertainment of type 2 diabetes
In the baseline questionnaire and all biennial follow-up questionnaires, participants were asked about the incidence of physician-diagnosed diabetes. Participants who reported such a diagnosis received a supplementary questionnaire querying about symptoms, diagnostic tests, and treatment for the purpose of confirmation. The self-report of diagnosis of type 2 diabetes has been demonstrated to be highly reliable in a validation study [26], in which self-reported diagnosis of diabetes was confirmed by medical records reviewed by an endocrinologist blinded to the supplementary questionnaire information for 61 of 62 randomly selected participants who responded. Only patients with confirmed type 2 diabetes were included as a case in the current diabetes case–control analysis. The American Diabetes Association 1998 criteria were used to confirm or refute self-reported type 2 diabetes diagnosis: (1) an elevated glucose concentration (fasting plasma glucose ≥7.0 mmol/l, random plasma glucose ≥11.1 mmol/l or plasma glucose ≥11.1 mmol/l at 2 h after an oral glucose load) and at least one symptom (excessive thirst, polyuria, weight loss or hunger) related to diabetes; (2) no symptoms, but elevated glucose concentrations on two occasions; (3) treatment with insulin or oral hypoglycaemic medication.
Statistical analysis
Distributions of continuous variables were assessed for normality, and natural log transformations of skewed biomarkers were used in subsequent analyses. Descriptive statistics for continuous variables were summarised as mean ± SE, and categorical variables were summarised using proportions according to alcohol consumption categories (0 g/day, >0 to <5 g/day, >5 to <15 g/day and ≥15 g/day).
General linear models were used to evaluate associations of alcohol consumption with plasma fetuin-A concentrations. Model 1 was adjusted for demographic information including age at blood draw (continuous), ethnicity (white/non-white), time of blood draw (blood collection cycle 1/2) and fasting status (yes/no). Model 2 was additionally adjusted for medical history variables including postmenopausal hormone use (yes/no), family history of diabetes (yes/no), history of hypertension (yes/no), and history of hypercholesterolaemia (yes/no). Model 3 was further adjusted for lifestyle variables including smoking status (current/former/never), physical activity (low/high), Alternative Healthy Eating Index (tertiles) [27] and total energy intake (continuous). Least-squares means (lsmeans) of fetuin-A were estimated in categories of alcohol consumption, and linear trends were tested. The Alternative Healthy Eating Index (tertiles) was generated excluding alcohol intake scores [27]. Sensitivity analysis stratified by two different blood collection cycles in the Nurses’ Health Study was assessed for the association under investigation. The merged overall analyses were conducted after confirmation of similar associations in the two cycles. Interaction was tested by adding an interaction term of alcohol consumption (continuous) with age (continuous), BMI (continuous), family history of diabetes (yes/no), hypertension (yes/no), hypercholesterolaemia (yes/no), Alternative Healthy Eating Index (continuous) and current smoker (yes/no), with adjustment for covariates in model 3. To assess the independent effects of specific alcohol types, the association of each alcohol type (i.e. wine, beer and spirits) with plasma fetuin-A was examined individually in multiple linear regression models, with mutual adjustment for individual types of alcohol. To account for variation in assay runs within the combined control samples, fetuin-A values were recalibrated as described by Rosner et al [28]. β coefficients from a linear regression model of fetuin-A with study assay run indicators were averaged. The difference between the corresponding β coefficient from the model and the average coefficient was subtracted from the unadjusted fetuin-A value to generate a standardised measurement.
To evaluate the contribution of fetuin-A to the association between alcohol consumption and type 2 diabetes, conditional logistic regression was used, matching for age at blood draw, ethnicity, time of blood draw and fasting status and adjusting for postmenopausal hormone use (yes/no), family history of diabetes (yes/no), history of smoking (yes/no), physical activity (quartiles), Alternative Healthy Eating Index (quartiles) and total energy intake (continuous). SAS macro %MEDIATE (publicly available at www.hsph.harvard.edu/faculty/spiegelman/mediate.html) was applied to estimate the proportion of the association explained by fetuin-A and other metabolic variables (i.e. CRP, BMI, fasting insulin, adiponectin, ALT and GGT), using 1 − (βmediator model/βbase model) × 100 according to the methods described by Lin et al [29].
For all statistical analyses, two-sided p < 0.05 was considered to be significant. All data analyses were performed using SAS software, version 9.3 for UNIX (SAS Institute, Cary, NC, USA).
Results
Characteristics of diabetes-free participants according to alcohol consumption categories are presented in Table 1. Participants who had a higher intake of alcohol were younger, more likely to smoke, and had higher adiponectin and lower BMI, Alternative Healthy Eating Index and HbA1c concentrations (all p for trend ≤0.02) (Table 1). In a subsample of diabetes-free individuals with fasting insulin data available (n = 470), fasting insulin concentrations tended to have non-linear U-shaped association with fetuin-A, although this was not significant: lsmean ± SE 45.1 ± 2.1 pmol/l for abstainers, 41.7 ± 2.1 pmol/l for 0.1–4.9 g/day consumers, 38.2 ± 2.8 pmol/l for 5.0–14.9 g/day consumers, and 43.1 ± 4.2 pmol/l for ≥15.0 g/day consumers. For the diabetes-free individuals (n = 1,331), median daily alcohol intake was 2 g/day (5th, 95th percentiles 0, 26).
Multiple regression models were constructed to assess whether alcohol consumption was associated with plasma fetuin-A concentration (Table 2). Higher alcohol consumption was associated with lower fetuin-A concentration after adjustment for demographic information, medical history and lifestyle variables (model 3; p for trend = 0.009). The inverse association of alcohol consumption with plasma fetuin-A remained significant after additional adjustment of model 3 with BMI or other biomarkers (i.e. CRP, adiponectin and HbA1c) (both p for trend ≤0.03) (Table 2). When model 3 was further adjusted for ALT and GGT concentrations, the association between alcohol consumption and fetuin-A remained significant (p for trend = 0.03). Among participants with ALT or GGT at above median (0.049 μkat/l [interquartile range 0.046, 0.053] for ALT and 0.050 μkat/l [interquartile range 0.043, 0.055] for GGT), the inverse association between alcohol consumption and fetuin-A remained significant with model 3 adjustment (both p for trend ≤0.004). In the subgroup of participants with waist circumference data (n = 749), a similar trend of the inverse association between alcohol consumption and fetuin-A was observed with adjustment for model 3 in addition to waist circumference as a continuous variable (p for trend = 0.06). When the association between alcohol consumption and fetuin-A was examined by time period of blood collection, a similar trend of inverse association was present in both cycles, although with attenuated significance (n = 861 and n = 470 for each period). When the highest category (≥15 g/day) of alcohol consumption was further divided into 15 to <30 g/day and ≥30 g/day consumer categories, the inverse association between alcohol consumption and fetuin-A remained with adjustment for model 3 covariates (p for linear trend = 0.0006). In addition, ≥30 g/day consumers had significantly lower fetuin-A concentrations than abstainers (p = 0.0004). When abstainers were removed from the analysis, the similar inverse trend association between alcohol consumption and fetuin-A remained in the three categories of drinkers with model 3 adjustment, although the significance was attenuated to non-significant (n = 926). Participants who develop diabetes by 2006 (n = 470) had a similar trend of inverse association between alcohol consumption and fetuin-A with model 3 adjustment, although not significant. When the subgroup of diabetes-free participants with fasting insulin measurements was stratified into tertiles of fasting insulin concentrations (n = 418), the significant association between alcohol and fetuin-A remained only in the highest tertile with model 3 adjustment (p for trend = 0.02) (p = 0.4 for interaction between fasting insulin and alcohol consumption). No effect modification of the association between alcohol intake and type 2 diabetes was observed for age, BMI, family history of diabetes, history of hypertension or hypercholesterolaemia, Alternative Healthy Eating Index or smoking.
To examine whether alcohol type (i.e. wine, beer and spirits) was independently associated with plasma fetuin-A, multiple regression models were constructed (Table 3). Wine was inversely associated with plasma fetuin-A (p for trend = 0.03) after adjustment for demographic information, medical history and lifestyle variables. However, these associations became attenuated to non-significance with mutual adjustment for individual types of alcohol (Table 3).
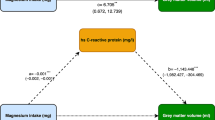
The proportions of the association between alcohol consumption and type 2 diabetes explained by metabolic variables were assessed in 470 diabetes case–control pairs (Table 4). The proportions of the association between alcohol consumption and type 2 diabetes that were independently explained were fetuin-A 18.4%, CRP 30.9%, BMI 33.2%, fasting insulin 54.8% and adiponectin 68.3% (all p for contribution <0.04), when 5 to <15 g/day alcohol consumers were compared with abstainers after adjustment for demographic information, medical history and lifestyle. ALT and GGT did not significantly contribute to the association between fetuin-A and diabetes (p for contribution =0.7 and =0.2, respectively).
Discussion
In a large population of middle-aged and older diabetes-free women, we observed that higher total alcohol consumption was associated with lower plasma fetuin-A. Wine was individually associated with plasma fetuin-A concentrations, but these associations were eliminated after mutual adjustment for individual types of alcohol. Therefore, the observed association is probably attributable to the total alcohol effect rather than an independent effect of specific types of alcohol. Fetuin-A and fasting insulin explain a significant proportion of the association between alcohol consumption and incident type 2 diabetes. Further, fasting insulin was a significant contributor to the association between fetuin-A and diabetes, while adiponectin did not contribute to this association in this population.
Similar to our findings, previous observational studies have demonstrated hepatic and glucose metabolic benefits of alcohol consumption [1, 2, 30–32]. Based on a meta-analysis of 20 prospective cohort studies, the association between alcohol intake and type 2 diabetes is U-shaped [1]. Alcohol consumption was found to be most protective when consumed at a level of 24 g/day for women and 22 g/day for men, while it was found to become harmful at levels above 50 g/day for women and 60 g/day for men [1]. Since our participants consumed a median of 2 (5th, 95th percentiles 0, 26) g/day of total alcohol, most women in this study probably consumed alcohol within the beneficial range. This explains the linear association observed between alcohol consumption and fetuin-A in the present investigation. Fetuin-A is a glycoprotein secreted by the liver and has recently emerged as a biomarker for type 2 diabetes [7–12]. Large-scale prospective studies have demonstrated that elevated plasma fetuin-A concentration is associated with an increased risk of developing type 2 diabetes [7–10]. Physiologically, this association may be explained by inhibition of insulin receptor tyrosine kinase activity by fetuin-A and therefore regulation of the downstream insulin signalling pathway [13–15]. Dysregulation of this process may subsequently result in impaired glucose metabolism leading to type 2 diabetes [13].
The underlying physiological explanation of the beneficial effect of moderate alcohol consumption on type 2 diabetes is not entirely clear. However, a number of observational and experimental studies have suggested that the mechanism may involve improving insulin sensitivity [4–6], potentially assisted by other biomarkers [5, 6, 33, 34]. Assessed using a hyperinsulinaemic–euglycaemic clamp, higher alcohol consumption was associated with improved insulin sensitivity in women [4]. In a randomised crossover trial of 26 women receiving 26 g/day alcohol for 3 weeks, this moderate alcohol intake increased concentrations of total and high-molecular-weight adiponectin [34]. Adiponectin has been found to correlate inversely with plasma fetuin-A in humans [16] and reported as a contributor to the association between alcohol consumption and diabetes [35]. Since adiponectin and fetuin-A are both involved in regulating the insulin signalling pathway [13, 17], we hypothesised that fetuin-A would be involved in the altered insulin signalling induced by alcohol consumption. In our study, in a large population of women, fetuin-A was a significant contributor to the association between alcohol intake and diabetes. It has been previously demonstrated that fasting insulin is a significant contributor to the association between fetuin-A and type 2 diabetes, whereas adiponectin is not a significant contributor to this association [7]. Therefore, population-based human data support previous animal and human in vitro experimental studies [13–15] showing that fetuin-A probably interacts directly with the insulin signalling pathway through the insulin receptor without the mediation of adiponectin. We demonstrated in our subsamples that the association between alcohol consumption and fetuin-A might be stronger in those diabetes-free women with higher concentrations of fasting insulin. However, this finding needs to be further investigated in a larger sample size.
Previous studies have reported the beneficial impact of alcohol consumption on type 2 diabetes [1, 2] and the association between fetuin-A and type 2 diabetes [7–10], but no large-scale human studies have investigated the association of alcohol consumption with fetuin-A. We show here for the first time to our knowledge that moderate alcohol consumption was associated with lower plasma fetuin-A in 1,331 diabetes-free women with fetuin-A measurements in addition to detailed characterisation of medical history and lifestyle.
This study, however, has limitations that should be noted. First, our study participants were female nurses of primarily European ancestry. We cannot generalise the present finding to men, given previously reported sex differences in drinking patterns and alcohol metabolism [36, 37] and sex interaction with fetuin-A in type 2 diabetes [11]. Since fetuin-A concentrations may vary among different ethnic groups [8], we also cannot generalise our results to all ethnic groups. Second, we cannot exclude the possibility that plasma fetuin-A measured in our study does not represent true, biologically relevant, long-term values. However, we have previously evaluated the stability of fetuin-A concentrations in blood samples collected 1–2 years apart [38] and determined a high intraclass correlation of 0.88 [7]. In the present alcohol and fetuin-A association analysis, our diabetes-free population was from combined control fetuin-A samples from three nested case–control studies. Although all blood sample processing and fetuin-A assays were performed under the internal Nurses’ Health Study protocol, this combining of control samples may have introduced additional measurement errors due to different batches of buffer solutions used or different technicians running the assays. To account for variation in assay runs, we recalibrated fetuin-A concentrations using previously published methods [28]. Third, fetuin-A may be more critical at specific stages of the disease progression. For example, it may be more relevant during the early stages in the absence of fatty liver or insulin resistance. However, we did not have information on non-alcoholic fatty liver disease diagnosis or insulin measurements from a big enough sample size to investigate this question further. In our sensitivity analysis in diabetes-free individuals, however, the association between alcohol consumption and fetuin-A remained significant with covariate adjustment for adiponectin, CRP or HbA1c. This suggests that the association may be present throughout the progression to diabetes. Fourth, we cannot conclude on the basis of this observation alone that fetuin-A is involved in the biological mechanism underlying beneficial effects of alcohol consumption on diabetes prevention. However, the null contribution of liver enzymes in the association between alcohol consumption and diabetes demonstrated in our study further supports a potential physiological role for fetuin-A and indicates the need for additional investigation on biological mechanisms underlying this association. Fifth, we had limited ability to assess the association in moderate to heavy alcohol consumers, since our participants were mostly consuming low to moderate levels of alcohol. Further, the causality of the association could not be drawn from this observational investigation. Since fetuin-A alone explained a small proportion of the association between alcohol consumption and diabetes, other related variables may be involved in the pathophysiological mechanisms. Therefore, additional investigations, including longitudinal analyses with larger sample size and biomarker measurements at multiple time points and well-designed randomised controlled trials, are needed to further confirm these associations.
In conclusion, moderate alcohol consumption is associated with lower plasma fetuin-A concentrations in middle-aged and older diabetes-free women. This association is attributed to total alcohol intake rather than independent effects of specific types of alcohol. Fetuin-A and fasting insulin explain a significant proportion of the association between alcohol consumption and incident type 2 diabetes in this population. These data contribute to emerging evidence of a pathophysiological involvement of fetuin-A in the progression to type 2 diabetes and enhance our current knowledge on the metabolic benefits of moderate alcohol consumption.
Abbreviations
- lsmean:
-
Least-squares mean
- FFQ:
-
Food Frequency Questionnaire
- CRP:
-
C-reactive protein
- ALT:
-
Alanine aminotransferase
- GGT:
-
γ-Glutamyl transpeptidase
References
Baliunas DO, Taylor BJ, Irving H et al (2009) Alcohol as a risk factor for type 2 diabetes. Diabetes Care 32:2123–2132
Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ (2005) Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care 28:719–725
Liangpunsakul S, Chalasani N (2012) What should we recommend to our patients with NAFLD regarding alcohol use? Am J Gastroenterol 107:976–978
Bonnet F, Disse E, Laville M et al (2012) Moderate alcohol consumption is associated with improved insulin sensitivity, reduced basal insulin secretion rate and lower fasting glucagon concentration in healthy women. Diabetologia 55:3228–3237
Sierksma A, Patel H, Ouchi N et al (2004) Effect of moderate alcohol consumption on adiponectin, tumor necrosis factor-alpha, and insulin sensitivity. Diabetes Care 27:184–189
Thamer C, Haap M, Fritsche A, Haering H, Stumvoll M (2004) Relationship between moderate alcohol consumption and adiponectin and insulin sensitivity in a large heterogeneous population. Diabetes Care 27:1240
Sun Q, Cornelis MC, Manson JE, Hu FB (2013) Plasma levels of fetuin-A and hepatic enzymes and risk of type 2 diabetes in women in the U.S. Diabetes 62:49–55
Ix JH, Wassel CL, Kanaya AM et al (2008) Fetuin-a and incident diabetes mellitus in older persons. JAMA 300:182–188
Ix JH, Biggs ML, Mukamal KJ et al (2012) Association of fetuin-a with incident diabetes mellitus in community-living older adults: the cardiovascular health study. Circulation 125:2316–2322
Stefan N, Fritsche A, Weikert C et al (2008) Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes 57:2762–2767
Laughlin GA, Barrett-Connor E, Cummins KM, Daniels LB, Wassel CL, Ix JH (2013) The sex-specific association of fetuin-A with type 2 diabetes in older community-dwelling adults: the Rancho Bernardo Study. Diabetes Care 36:1994–2000
Rasul S, Wagner L, Kautzky-Willer A (2012) Fetuin-A and angiopoietins in obesity and type 2 diabetes mellitus. Endocrine 42:496–505
Goustin A-S, Abou-Samra AB (2011) The “thrifty” gene encoding Ahsg/Fetuin-A meets the insulin receptor: insights into the mechanism of insulin resistance. Cell Signal 23:980–990
Auberger P, Falquerho L, Contreres JO et al (1989) Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity. Cell 58:631–640
Srinivas PR, Wagner AS, Reddy LV et al (1993) Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol Endocrinol 7:1445–1455
Hennige AM, Staiger H, Wicke C et al (2008) Fetuin-A induces cytokine expression and suppresses adiponectin production. PLoS ONE 3:e1765
Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K (2006) Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116:1784–1792
Prentice RL, Breslow NE (1978) Retrospective studies and failure time models. Biometrika 65(1):153–158
Willett WC, Sampson L, Stampfer MJ et al (1985) Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 122:51–65
Willett W (1998) Nutritional epidemiology. Oxford University Press, New York
Fuchs CS, Stampfer MJ, Colditz GA et al (1995) Alcohol consumption and mortality among women. N Engl J Med 332:1245–1250
Giovannucci E, Colditz G, Stampfer MJ et al (1991) The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol 133:810–817
Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Hennekens CH, Speizer FE (1987) Moderate alcohol consumption and the risk of breast cancer. N Engl J Med 316:1174–1180
Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC (1990) Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1:466–473
Colditz GA, Martin P, Stampfer MJ et al (1986) Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol 123:894–900
Manson JE, Stampfer MJ, Colditz GA et al (1991) Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 338:774–778
McCullough ML, Feskanich D, Stampfer MJ et al (2002) Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 76:1261–1271
Rosner B, Cook N, Portman R, Daniels S, Falkner B (2008) Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol 167:653–666
Lin DY, Fleming TR, de Gruttola V (1997) Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med 16:1515–1527
Dunn W, Xu R, Schwimmer JB (2008) Modest wine drinking and decreased prevalence of suspected nonalcoholic fatty liver disease. Hepatology 47:1947–1954
Gunji T, Matsuhashi N, Sato H et al (2009) Light and moderate alcohol consumption significantly reduces the prevalence of fatty liver in the Japanese male population. Am J Gastroenterol 104:2189–2195
Moriya A, Iwasaki Y, Ohguchi S et al (2011) Alcohol consumption appears to protect against non-alcoholic fatty liver disease. Aliment Pharmacol Ther 33:378–388
Englund Ögge L, Brohall G, Behre CJ, Schmidt C, Fagerberg B (2006) Alcohol consumption in relation to metabolic regulation, inflammation, and adiponectin in 64-year-old Caucasian women: a population-based study with a focus on impaired glucose regulation. Diabetes Care 29:908–913
Joosten MM, Witkamp RF, Hendriks HFJ (2011) Alterations in total and high-molecular-weight adiponectin after 3 weeks of moderate alcohol consumption in premenopausal women. Metabolism 60:1058–1063
Beulens JWJ, Rimm EB, Hu FB, Hendriks HFJ, Mukamal KJ (2008) Alcohol consumption, mediating biomarkers, and risk of type 2 diabetes among middle-aged women. Diabetes Care 31:2050–2055
Nielsen SJ, Kit BK, Fakhouri T, Ogden CL (2012) Calories consumed from alcoholic beverages by U.S. adults, 2007–2010. NCHS Data Brief 110:1–8
Chrostek L, Jelski W, Szmitkowski M, Puchalski Z (2003) Gender-related differences in hepatic activity of alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase in humans. J Clin Lab Anal 17:93–96
Kotsopoulos J, Tworoger SS, Campos H et al (2010) Reproducibility of plasma and urine biomarkers among premenopausal and postmenopausal women from the Nurses’ Health Studies. Cancer Epidemiol Biomarkers Prev 19:938–946
Funding
This study was supported by research grants (CA87969, CA49449, DK58845, DK58785, P30 DK46200, HL088521 and HL34594) from the National Institutes of Health. SHL was supported by a Canadian Institutes of Health Research Fellowship Award. The funders have no role in the design and conduct of the study, collection, analysis and interpretation of the data, and reparation and approval of the manuscript.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
SHL and FBH participated in study concept, design, statistical analysis and interpretation. SHL drafted the article, and all authors participated in interpretation of the data, critical revision and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ley, S.H., Sun, Q., Jimenez, M.C. et al. Association between alcohol consumption and plasma fetuin-A and its contribution to incident type 2 diabetes in women. Diabetologia 57, 93–101 (2014). https://doi.org/10.1007/s00125-013-3077-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-3077-8