Abstract
Aims/hypothesis
This study aimed to investigate whether variation in long-term glycaemia in type 1 diabetes as measured by HbA1c variability is associated with the cumulative incidence and risk of retinopathy requiring laser treatment.
Methods
The effect of HbA1c variability was assessed in 2,019 Finnish Diabetic Nephropathy (FinnDiane) study patients. The patients were studied in two partially overlapping subcohorts with either verified first laser treatment (n = 1,459) or retinopathy severity graded from ophthalmic records with the Early Treatment of Diabetic Retinopathy Study (ETDRS) scale (n = 1,346). The ratio of intrapersonal SD and mean of serially measured HbA1c was considered an estimate of HbA1c variability.
Results
A subcohort of 1,459 patients did not have laser treatment prior to the first FinnDiane visit and 174 of these patients were treated during a mean follow-up period of 5.2 ± 2.2 years. The 5 year cumulative incidence of laser treatment was 19% (95% CI 15, 24) in the highest quartile of HbA1c variability and 10% (95% CI 7, 12) in the lowest quartile (p < 0.001, Gray’s test) with a corresponding HR of 1.6 (95% CI 1.1, 2.5; p = 0.02) adjusted for renal status, diabetes duration, mean HbA1c, blood pressure, sex and number of HbA1c measurements. In a subcohort of 1,346 patients, 434 patients had proliferative diabetic retinopathy (PDR). Patients in the highest quartile of HbA1c variability had an increased risk of PDR compared with the lowest quartile (HR 1.7 [95% CI 1.3, 2.2]; p < 0.001]).
Conclusions/interpretation
HbA1c variability was associated with an increased cumulative incidence and risk of retinopathy requiring laser treatment in type 1 diabetes.
Similar content being viewed by others
Introduction
After 20 years of diabetes almost all patients with type 1 diabetes show signs of retinopathy, and severe visual loss eventually threatens 7–12% of the patients [1]. The most common indication for laser treatment in type 1 diabetes is proliferative diabetic retinopathy (PDR). PDR is also the most severe form of retinopathy; without any treatment most of the patients with this complication are at risk for becoming blind after 5–10 years [2]. The incidence of PDR rises almost linearly after 10 years of diabetes and roughly one-third of patients have PDR after 25 years of diabetes [3].
Important risk factors for PDR are duration of diabetes and glycaemic control. Poor glycaemic control increases not only the incidence but also the progression of retinopathy. Male sex and high BP may further increase the risk for retinopathy [1], and genetic factors are also likely to play a role [4]. Despite identical mean HbA1c values, patients may show a wide variation in their long-term glycaemic profile. Data from the DCCT indicate that variability in long-term glycaemia, defined as intrapersonal SDs of quarterly measured HbA1c, is a risk factor for a three-step progression of retinopathy on the Early Treatment of Diabetic Retinopathy Study (ETDRS) 12 step severity scale [5]. Whether HbA1c variability also affects the development of proliferative retinopathy and the requirement for subsequent laser treatment is not known. We have recently shown that HbA1c variability predicts both the development and progression of diabetic nephropathy [6]. Given the close relationship between diabetic nephropathy and proliferative retinopathy, it is likely that HbA1c variability is also a risk factor for PDR.
The aim of this study was to investigate whether long-term variation in glycaemia, as measured by HbA1c variability, is associated with an increased risk of severe retinopathy requiring laser treatment.
Methods
The present study was undertaken as part of the ongoing observational Finnish Diabetic Nephropathy (FinnDiane) study, which has, since 1997, collected comprehensive data from patients with type 1 diabetes. Participating study centres are diabetes and renal outpatient clinics at all five university central hospitals, all 16 central hospitals, the majority (n = 27) of regional hospitals, and 31 major primary healthcare centres in Finland. All adult patients with type 1 diabetes at these centres have been invited to participate and 78% have responded positively [7]. To date, the FinnDiane study has 4,800 patients with type 1 diabetes; the geographic distribution of the patients closely follows the distribution of the general population in Finland. All study patients gave their written, informed consents and the study protocol is in accordance with the Declaration of Helsinki, and was approved by the ethics committee of the Helsinki University Central Hospital.
C-peptide-negative patients with age at onset <40 years and insulin treatment initiated within 1 year of diagnosis were considered to have type 1 diabetes. The proportion of patients with C-peptide concentration <0.033 nmol/l, which represents the detection limit of the assay (Human C-peptide RIA Kit, Linco Research, MO, USA), was 82.3%. Patients with age at onset >35 years were included as we have shown that the FinnDiane patients with age at onset >35 years have a similar distribution of risk/protective genotypes for type 1 diabetes as those patients with age at onset <20 years [8]. Data on medication, cardiovascular status, diabetic complications and hypertension were obtained using a standardised questionnaire completed by the patient’s attending physician. Patients’ BP was measured twice in the sitting position using a mercury sphygmomanometer after a rest of at least 10 min. Mean arterial BP (MAP) was calculated according to the formula: MAP = diastolic BP + 1/3(systolic BP − diastolic BP). Anthropometric data, such as height, weight and waist and hip circumferences, were recorded, and blood was drawn for the laboratory measurements, including HbA1c and C-reactive protein (CRP) [9]. HbA1c was measured at the local study centres with standardised assays (normal range 4.0–6.0%). The nationwide validity of HbA1c measurements in Finland has previously been reported to show a high correlation with the DCCT reference method [10]. As a normalised measure of variability, the CV for HbA1c was calculated as the ratio of intrapersonal SD and mean to correct for larger SD because of higher absolute values of HbA1c. The patients were then divided into quartiles of HbA1c variability (CV) in both PDR and laser-treatment subcohorts for further analyses. The results shown in this study are based on the CV quartiles for easier interpretability and comparability with our previous publication [6].
Data on all-cause mortality were obtained until 24 March 2009 from the Population Register Centre of Finland. The renal status was defined based on the AER in at least two out of three consecutive overnight or 24 h urine collections. Patients were divided by renal status into four categories: normal AER (<20 μg/min or <30 mg/24 h); microalbuminuria (AER ≥20 and <200 μg/min or ≥30 and <300 mg/24 h); and macroalbuminuria (AER ≥200 μg/min or ≥300 mg/24 h). In addition, patients were considered to have end-stage renal disease (ESRD) if they had received a kidney transplant or if they were undergoing dialysis treatment. Diabetic nephropathy (yes/no) was defined as macroalbuminuria or ESRD.
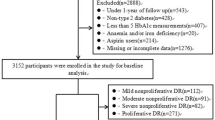
The patient data were analysed in two subcohorts with 786 overlapping patients in order to improve the statistical power (Fig. 1). The laser-treatment subcohort consisted of 1,459 patients followed up for the occurrence of first laser treatment. Since 2004, follow-up data have been collected in a consecutive order for a high number of FinnDiane patients either by re-examination of the patients or by review of the medical files. The collection of follow-up data started from the patients who were the first to be recruited to the FinnDiane study. In January 2012 the FinnDiane database comprised 2,019 patients with follow-up data on whether the patient had been laser treated (yes/no) as verified from medical files by attending physician, data on year of the first laser treatment and data on at least two HbA1c measurements 1 year apart. Of the 2,019 patients, 1,459 did not have any prior laser treatment episodes before the first FinnDiane visit. Patients who were not included in the subcohort of 1,459 patients had either been laser treated before the first FinnDiane visit, or the ophthalmic data and/or serial HbA1c measurements were insufficient to construct a follow-up period after the first FinnDiane visit. The year of the first laser treatment was used in the statistical analysis.
The risk factors for proliferative retinopathy were further analysed in a subcohort of 1,346/2,019 patients (PDR subcohort) with detailed longitudinal ophthalmic data available on retinopathy progression as measured on the ETDRS scale. The patients in this subcohort were mostly those who were the earliest to have been recruited to the FinnDiane study. However, this subcohort also included almost 400 patients from families with at least two diabetes patients, some of whom may have been recruited at a later stage [4]. We obtained fundus photographs taken for screening or documentation purposes and/or records of dilated slit-lamp fundus examinations performed by a specialist in ophthalmology for these 1,346 patients. Records of fundus examinations by ophthalmologists were available for 1,076/1,346 (80%) patients and fundus photographs were available for 1,052/1,346 (78%) of the patients. The patients had been photographed on a median of three occasions (interquartile range [IQR] 1–5) per patient. All the patients who were not examined by ophthalmologists had had serial fundus photographs. Both ophthalmic records and photographs were available for 782/1,346 (58%) patients. All available data were used to score the severity and progression of retinopathy, a procedure handled by an ophthalmologist (K. Hietala) unaware of the demographic data and the presence or absence of other complications. The ETDRS grading scale, where ten represents no retinopathy, and 61 and upwards represents proliferative retinopathy, was used [11]. The eye with the more severe retinopathy was used to assess severity. Laser treatment and laser scars in fundus photographs were not taken as the only evidence of PDR as severe background retinopathy and macular oedema are also indications for laser treatment. Because laser treatment is considered a surgical procedure in Finland, the indications for performing it are meticulously stated in the ophthalmic records and could therefore be verified for each individual treatment episode and treated eye. There were a total of 11 (IQR 7–15) laser treatment episodes per patient. Of the 434 patients with PDR 170 (39%) were initially laser treated for indications other than PDR. Of the patients in the PDR subcohort, 81.1% who were laser treated were eventually diagnosed with PDR. The laser-treatment cohort is entirely prospective, whereas the PDR cohort is mostly retrospective. The majority of the PDR diagnoses (302/434 [69.6%]) were made before the first FinnDiane visit and the highest attained ETDRS scores for all patients in the PDR subcohort (n = 1,346) were recorded at a median of 0.3 (IQR 5.9 to −2.9) years after the first FinnDiane visit.
Statistical analyses
Except for the serial HbA1c measurements, the laboratory and anthropometric measurements taken at the first FinnDiane visit were used in all the statistical analyses. Data are presented as means ± SD for continuous normally distributed variables, and median and IQR for non-normally distributed variables. The 95% CIs are given for estimates of cumulative incidences, HRs and ORs. Differences between groups were compared with one-way ANOVA (Tukey). Trends in normally distributed variables were analysed with linear polynomial contrasts (ANOVA), and trends in non-normally distributed variables were analysed with the Jonckheere–Terpstra test. Spearman’s ρ (r s) was used as a measure of correlation. Cumulative incidence for the prospectively followed subcohort was calculated with the cumulative incidence function, which correctly accounts for the competing risk for death, and the differences in cumulative incidence were tested with Gray’s test [12]. As the cumulative incidences of death in CV quartiles were borderline different in the laser-treatment subcohort (p = 0.09, Gray’s test) and significantly different in the PDR subcohort (p = 0.05, Gray’s test), a recently published modification of the Fine and Gray competing-risks regression for clustered data (participating study centres) was used to calculate the risk for laser treatment and PDR [13]. The Fine and Gray model takes into account the competing risk for death. The failure time for the Fine and Gray model was calculated as the duration of diabetes from the first FinnDiane visit to first laser treatment in the laser-treatment subcohort and in the PDR as diabetes duration from onset of diabetes to PDR. The effect modification (i.e. whether the effect of a certain variable on the risk for PDR or laser treatment varied according to the level of another covariate) was tested for by adding corresponding interaction terms to the regression models. Pairwise first- and second-order interactions between mean HbA1c, CV and duration of diabetes were studied. None of the interaction terms was significant. The plotted Schoenfeld residuals did not indicate any time-varying effects for the covariates. Model selection was then based on Bayesian information criteria (BIC). All statistical calculations were performed with SPSS 15.0 (SPSS, Chicago, IL, USA), except the cumulative incidence function, Gray’s test and Fine and Gray regression model, for which R open source software (www.r-project.org) was used.
Results
The intrapersonal mean of serially measured HbA1c was 8.4 ± 1.2% for all patients (n = 2,019), with ten (IQR 3–18) measurements per patient. There was a clear correlation between the first single-measurement HbA1c and the mean of serial HbA1c (r s 0.77; p < 0.001). The male/female ratio was 995/1,024. Mean age at onset of type 1 diabetes was 15.3 ± 9.2 years and mean duration of diabetes was 22.9 ± 11.9 years.
There were a total of 1,459 patients with no previous laser treatment and with prospective follow-up data after the first FinnDiane visit for a period of 5.2 ± 2.2 years. Of these patients, 174 had had their first laser treatment during the follow-up period (Table 1). The estimated 5 year cumulative incidence of laser treatment, accounting for the competing risk for death, was: 19% (95% CI 15, 24) in the fourth quartile of HbA1c variability; 12% (95% CI 9, 16) in the third quartile; 9% (95% CI 6, 12) in the second quartile; and 10% (95% CI 7, 13) in the first quartile (p < 0.001, Gray’s test) (Fig. 2). The patients in the fourth quartile of HbA1c variability had the highest mean HbA1c (8.6 ± 1.4%), the highest triacylglycerol (1.0 mmol/l [IQR 0.7–1.3]) and the lowest age at onset of type 1 diabetes (15.3 ± 9.2 years) (Table 1). Mortality and CRP were also higher in patients with a wide HbA1c variability. Total cholesterol, BP and body mass index were no different in higher quartiles of variability. In a Fine and Gray regression model, adjusted for clinically significant risk factors (duration of diabetes prior to first FinnDiane visit, renal status, mean HbA1c, MAP, sex and number of HbA1c measurements), the quartile of HbA1c variability was a significant overall risk factor for retinopathy requiring laser treatment (p = 0.04, Wald test). The highest adjusted risk for retinopathy requiring laser treatment (HR 1.6 [95% CI 1.1, 2.5]; p = 0.02) was seen in the fourth quartile compared with the first quartile of HbA1c variability.
Cumulative incidence estimates for laser treatment in different quartiles of HbA1c variability (CV) (n = 1,459; p < 0.001, Gray’s test) accounting for the competing risk for death. Solid line, first quartile; dashed line, second quartile; dotted line, third quartile; dashed/dotted line, fourth quartile
The associations between conventional risk factors, HbA1c variability and PDR were further analysed in a subcohort of patients (n = 1,346) with retinopathy status and specific indications for laser treatment verified from ophthalmic records and fundus photographs (Tables 2 and 3). Of the 1,346 patients with verified ophthalmic status, 434 (32%) had been diagnosed with PDR and all of these patients had been laser treated. Prominent features of patients with PDR were lower than average age at onset of type 1 diabetes (11.0 ± 7.2) years, higher mean HbA1c 8.7 ± 1.2% (72 ± 14.2 mmol/mol), higher MAP (102.2 ± 12 mmHg), higher body mass index (25.6 ± 3.9 kg/m2) and longer duration of diabetes (33.3 ± 9.0 years). Patients with PDR also had higher than average values of CRP and triacylglycerol as well as lower C-peptide values compared with patients with no retinopathy. The patient’s highest attained ETDRS score and the renal status showed a significant correlation (r s 0.64; p < 0.001). In a Fine and Gray regression model, HbA1c variability quartile was significantly associated with PDR (p = 0.003, Wald test) (Table 3). The fourth quartile of HbA1c variability had the highest HR 1.7 (95% CI 1.3, 2.2; p < 0.001), compared with the first quartile. As expected for the other covariates, mean HbA1c (HR 1.2 [95% CI 1.1, 1.3; p < 0.001]) and BP (HR 1.02 [95% CI 1.01, 1.03; p < 0.001]), were significantly associated with PDR. However, male sex was not a significant factor (HR 1.1 [95% CI 0.9, 1.3; p = 0.5]). Because of the strong correlation between renal status and the ETDRS score (r s 0.64; p < 0.001), the renal status was not included in the Fine and Gray regression model for PDR (Table 3). However, if only patients with no nephropathy (n = 969) were included in the regression model, HbA1c variability above the median was still associated with PDR (HR 1.4 [95% CI 1.01, 2.0]; p = 0.04]).
High mean HbA1c correlated positively with the intrapersonal SD of HbA1c values (r s 0.39; p < 0.001). Thus, the patients with the highest mean HbA1c values had the highest variation in HbA1c values. The number of HbA1c measurements also correlated positively with the intrapersonal SD of HbA1c values (r s 0.32; p < 0.001). Because of these correlations and the uneven HbA1c measurement intervals we selected the first measured HbA1c value of each year, one value per year and per patient, thus standardising the measurement intervals by re-sampling to 1 year for every patient. This reduced the number of measurements from ten (IQR 3–18) to five (IQR 2–8) per patient. The CV was then recalculated and the patients were again divided into quartiles of variability. This produced HR 1.8 (95% CI 1.03, 3.03; p = 0.04) for retinopathy requiring laser treatment and HR 1.4 (95% CI 1.03, 1.8; p = 0.03) for proliferative retinopathy in the fourth quartile vs first quartile. Furthermore, an alternative adjustment for the number of HbA1c measurements was explored by using the SD of \( {{{\mathrm{Hb}{{\mathrm{A}}_{{1\mathrm{c}}}}}} \left/ {{\surd \left[ {{n \left/ {{\left( {n - 1} \right)}} \right.}} \right]}} \right.} \) as a measure of HbA1c variability. The HbA1c variability was a significant risk factor with this adjustment as well as without any adjustments in both subcohorts.
Discussion
We found an increased need for laser treatment in patients in the highest quartile of HbA1c variability as well as an association between PDR and HbA1c variability. The estimates of 5 year cumulative incidence of laser treatment were 19% in the highest quartile of HbA1c variability compared with 10% in the lowest quartile, with a 1.6-fold higher adjusted risk for retinopathy requiring laser treatment. Proliferative retinopathy is the most common indication for laser treatment in type 1 diabetes. In a competing-risks regression analysis of risk factors specific for PDR, the highest risk (HR 1.7) was seen in the highest variability quartile compared with the lowest quartile. These observations are in line with previous evidence from the DCCT, which showed that long-term variations in glycaemia as measured by HbA1c variability predicted a three-step progression on the 12-step ETDRS scale [5]. The present study adds to this by showing that retinopathy worsening leads to significant clinical consequences, the increased need for laser treatment.
Many different mechanisms have been suggested to explain the increased risk for microvascular complications in patients with high HbA1c variability. One plausible explanation is the exponential fashion by which both higher mean HbA1c and higher HbA1c variability increase the risk for microvascular complications [5]. Thus, even short periods of higher HbA1c could significantly increase the risk for PDR, even in the presence of comparable mean HbA1c levels. The cumulative incidence of laser treatment was clearly different between the highest quartile and the lowest two quartiles of HbA1c variability, which could result from the exponentially rising risk. However, the inclusion of first- and second-order interaction terms in the regression models to account for the exponential increase in risk with higher mean HbA1c did not change the results in Fine and Gray regression models. Furthermore, the ETDRS score and the mean HbA1c did not show an exponential relationship, which suggests that other mechanisms are also likely to have a role. The possible impact of HbA1c fluctuation itself is supported by the fact that short-term worsening of retinopathy has been observed in patients with improved glycaemic control [14]. It could be hypothesised that recurrent episodes of good and poor glycaemic control lead to a more sustained progression of retinopathy. Interestingly, short-term glucose fluctuations do not appear to increase the risk for retinopathy progression [15]. The difference could be related to ‘metabolic memory’, in which even periods of short hyperglycaemia place patients at higher risk [16]. Furthermore, HbA1c shows biological variability, independent of mean blood glucose, that may be related to the formation of AGE [17]. There are many cellular mechanisms that may be involved, such as induction of the growth factors IGF-1 and vascular endothelial growth factor (VEGF) [18, 19] and impaired endothelial function [20] as well as other detrimental in vitro cellular effects [21–23]. In addition, HbA1c variability has been associated with insulin resistance, which in itself has been implicated in the pathogenesis of diabetic complications [24]. In line with this, we noted higher triacylglycerols in patients in the highest quartile of HbA1c variability. This supports the possible association with insulin resistance, although the inclusion of triacylglycerol as a surrogate marker for insulin resistance in the models did not change the overall significance of HbA1c variability in either subcohort. Although hyperglycaemic exposure is a prerequisite for PDR, the factors that actually trigger the angiogenic switch remain to be elucidated. One important factor may be retinal hypoxia [25].
Interestingly, in this study the patients with the lowest HbA1c variability had the highest age at onset of type 1 diabetes. It could therefore be speculated that the lower HbA1c variability could at least partially contribute to the lower risk for PDR in patients with higher age at onset of type 1 diabetes [3]. In previous studies, diabetes onset after puberty has been linked to a less aggressive form of type 1 diabetes [26] and it has also been observed that beta cells are better preserved when type 1 diabetes begins in adulthood [27]. The DCCT data indicate that patients with any residual C-peptide secretion, and especially those with the highest stimulated concentrations, have a reduced incidence of retinopathy and nephropathy [28]. Thus, the preservation of beta cells could explain the connection between lower risk for PDR and less variable HbA1c measurements in patients with higher age at onset of type 1 diabetes. The patients with PDR in the present study had the lowest C-peptides, which supports this hypothesis. However, the patients in the highest quartile of HbA1c variability had, in fact, the highest C-peptide level, which may be due to the relatively short duration of diabetes of these patients.
The main strength of this study is the large sample size, the accurate timing of laser treatment and PDR for the respective subcohorts and the fact that renal status could explicitly be taken into account as a confounding factor in the statistical analyses. Furthermore, the results of this study reflect a normal clinical setting without any confounding interventions for HbA1c. A high percentage of patients with diabetes in Finland are undergoing regular fundus photography and the majority of the patients in this study had attended several screening examinations. The national guidelines for the screening of diabetic retinopathy were published already in 1992 and updated in 2006 [29]. The timing and diagnosis of PDR is reliable, because it was based on a combination of several dilated slit-lamp fundus examinations by ophthalmologists and/or serial fundus photographs [30]. Only in 55/434 (13%) patients was PDR diagnosed at the first clinical fundus examination. Thus, there were no available reference points for these patients before they had developed PDR.
There are some limitations to the present study. The serial HbA1c values were collected from the laboratory records available from the patient’s routine clinical follow-up. Thus, there were no pre-specified intervals between HbA1c measurements, and also the number of measurements per individual patient varied. The number of HbA1c measurements correlated positively with the intrapersonal SD of HbA1c (r s 0.32), which could result from the repeated attempts by the attending physicians to improve the glycaemic control of the patients with high HbA1c values. The regression models were therefore adjusted for both the number of HbA1c measurements and mean HbA1c. Moreover, standardisation of the HbA1c measurement intervals to 1 year by re-sampling of the original measurements retained the significance for variability quartile as a risk factor for both PDR and laser treatment. As 69% of the patients developed PDR even before the first serially measured HbA1c value, the assumption of no change in the prognostic variable CV over time is not as valid in the PDR subcohort as in the entirely prospective laser-treatment subcohort. Although unlikely, the CV may thus have been affected by PDR. Another potential limitation is the use of HbA1c measured at the local study centres, not at a central laboratory. This should not influence the intrapersonal variability of HbA1c though, because the measurements were performed at the same centre and Fine and Gray models accounted for the possible clustering effect within participating study centres. The FinnDiane study is not, by strict definition, a population-based study, which may limit the overall generalisability of the results. However, possible selection bias is unlikely as the geographic distribution of the FinnDiane patients closely follows the distribution of the general population and the high response rate makes significant biases unlikely. Furthermore, the treatment of diabetes and its complications is uniform across Finland. An attempt was made to take into account all possible confounding factors, but as the data are observational it is possible that there are unknown factors left that may modify both retinopathy and HbA1c variability.
In conclusion, we found an increased need for laser treatment in patients with type 1 diabetes who were in the highest quartile of HbA1c variability. Furthermore, there was an association between HbA1c variability and PDR, which suggests that HbA1c variability may also play a role in the development of PDR.
Abbreviations
- CRP:
-
C-reactive protein
- ETDRS:
-
Early Treatment of Diabetic Retinopathy Study
- FinnDiane:
-
Finnish Diabetic Nephropathy
- IQR:
-
Interquartile range
- MAP:
-
Mean arterial BP
- PDR:
-
Proliferative diabetic retinopathy
References
Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE (2008) The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology 115:1859–1868
Deckert T, Simonsen SE, Poulsen JE (1967) Prognosis of proliferative retinopathy in juvenile diabetics. Diabetes 16:728–733
Hietala K, Harjutsalo V, Forsblom C, Summanen P, Groop PH (2010) Age at onset and the risk of proliferative retinopathy in type 1 diabetes. Diabetes Care 33:1315–1319
Hietala K, Forsblom C, Summanen P, Groop PH (2008) Heritability of proliferative diabetic retinopathy. Diabetes 57:2176–2180
Kilpatrick ES, Rigby AS, Atkin SL (2008) A1C variability and the risk of microvascular complications in type 1 diabetes: data from the Diabetes Control and Complications Trial. Diabetes Care 31:2198–2202
Waden J, Forsblom C, Thorn LM, Gordin D, Saraheimo M, Groop PH (2009) A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes 58:2649–2655
Fagerudd J, Forsblom C, Pettersson-Fernholm K, Groop PH (2004) Implementation of guidelines for the prevention of diabetic nephropathy. Diabetes Care 27:803–804
Andersen MK, Lundgren V, Turunen JA et al (2010) Latent autoimmune diabetes in adults differs genetically from classical type 1 diabetes diagnosed after the age of 35 years. Diabetes Care 33:2062–2064
Saraheimo M, Teppo AM, Forsblom C, Fagerudd J, Groop PH (2003) Diabetic nephropathy is associated with low-grade inflammation in type 1 diabetic patients. Diabetologia 46:1402–1407
Penttila IM, Halonen T, Punnonen K, Tiikkainen U (2005) Best use of the recommended IFCC reference method, material and values in HbA1C analyses. Scand J Clin Lab Invest 65:453–462
Davis MD, Fisher MR, Gangnon RE et al (1998) Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: early Treatment Diabetic Retinopathy Study Report #18. Invest Ophthalmol Vis Sci 39:233–252
Scrucca L, Santucci A, Aversa F (2007) Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transpl 40:381–387
Zhou B, Fine J, Latouche A, Labopin M (2012) Competing risks regression for clustered data. Biostatistics 13:371–383
Helve E, Laatikainen L, Merenmies L, Koivisto VA (1987) Continuous insulin infusion therapy and retinopathy in patients with type I diabetes. Acta Endocrinol (Copenh) 115:313–319
Kilpatrick ES, Rigby AS, Atkin SL (2006) The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care 29:1486–1490
DCCT Group (2000) Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med 342:381–389
McCarter RJ, Hempe JM, Gomez R, Chalew SA (2004) Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care 27:1259–1264
Chantelau E (1998) Evidence that upregulation of serum IGF-1 concentration can trigger acceleration of diabetic retinopathy. Br J Ophthalmol 82:725–730
Poulaki V, Qin W, Joussen AM et al (2002) Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1alpha and VEGF. J Clin Invest 109:805–815
Ceriello A, Esposito K, Piconi L et al (2008) Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 57:1349–1354
Jones SC, Saunders HJ, Qi W, Pollock CA (1999) Intermittent high glucose enhances cell growth and collagen synthesis in cultured human tubulointerstitial cells. Diabetologia 42:1113–1119
Quagliaro L, Piconi L, Assaloni R et al (2005) Intermittent high glucose enhances ICAM-1, VCAM-1 and E-selectin expression in human umbilical vein endothelial cells in culture: the distinct role of protein kinase C and mitochondrial superoxide production. Atherosclerosis 183:259–267
Monnier L, Mas E, Ginet C et al (2006) Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 295:1681–1687
Groop PH, Forsblom C, Thomas MC (2005) Mechanisms of disease: pathway-selective insulin resistance and microvascular complications of diabetes. Nat Clin Pract Endocrinol Metab 1:100–110
Simo R, Carrasco E, Garcia-Ramirez M, Hernandez C (2006) Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr Diabetes Rev 2:71–98
Kordonouri O, Danne T, Enders I, Weber B (1998) Does the long-term clinical course of type I diabetes mellitus differ in patients with prepubertal and pubertal onset? Results of the Berlin Retinopathy Study. Eur J Pediatr 157:202–207
Karjalainen J, Salmela P, Ilonen J, Surcel HM, Knip M (1989) A comparison of childhood and adult type I diabetes mellitus. N Engl J Med 320:881–886
Steffes MW, Sibley S, Jackson M, Thomas W (2003) Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 26:832–836
Summanen P, Kallioniemi V, Komulainen J et al (2007) Diabetic retinopathy. Current care guideline. Duodecim 123:421–422
Hutchinson A, McIntosh A, Peters J et al (2000) Effectiveness of screening and monitoring tests for diabetic retinopathy—a systematic review. Diabet Med 17:495–506
Acknowledgements
We acknowledge all the physicians and nurses at each centre participating in the collection of patients (see electronic supplementary material [ESM]).
Funding
This study was supported by grants from the Folkhälsan Research Foundation, Wilhelm and Else Stockmann Foundation, Finnish Eye Foundation, European Commission, Medicinska Understödsföreningen Liv och Hälsa, Signe and Ane Gyllenberg Foundation and the Waldemar von Frenckell Foundation, and by an EVO governmental grant (TYH 3263).
Duality of interest
P.-H. Groop has received speaker honoraria from Boehringer Ingelheim, Cebix,Lilly, Genzyme, MSD, Novartis and Novo Nordisk. P.-H. Groop is an advisory board memberof Boehringer Ingelheim, Novartis and Cebix. P. Summanen has received speaker honoraria from Alcon, Abbott, Lilly, MSD, Novo Nordisk and Santen. K. Hietala has received a speaker honorarium from Orion. J. Kytö has worked as medical adviser for Pfizer.
Contribution statement
KH, JW, CF and P-HG contributed to the study concept and design. KH, JW, CF, VH, JK, PS and P-HG contributed to acquisition of data. Data analyses were performed by KH, who also wrote the initial draft of the paper. KH, JW, CF, VH, JK, PS and P-HG revised the paper for important intellectual content. All authors approved the final version of the paper.
Author information
Authors and Affiliations
Consortia
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 19 kb)
Rights and permissions
About this article
Cite this article
Hietala, K., Wadén, J., Forsblom, C. et al. HbA1c variability is associated with an increased risk of retinopathy requiring laser treatment in type 1 diabetes. Diabetologia 56, 737–745 (2013). https://doi.org/10.1007/s00125-012-2816-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-012-2816-6