Abstract
Aims/hypothesis
It is difficult to use HbA1c as an indicator of glycaemic control in patients with neonatal diabetes mellitus (NDM) because of high levels of fetal haemoglobin (HbF) remaining in the blood. In this study, glycated albumin (GA), which is not affected by HbF, and HbA1c were compared to evaluate whether they reflect glycaemic control in patients with NDM.
Methods
This study included five patients with NDM. Age at diagnosis was 38 ± 20 days. Insulin therapy was started in all patients, and levels of GA, HbA1c and HbF were measured monthly for 6 months. One-month average preprandial plasma glucose (aPPG) was calculated using self-monitoring of blood glucose.
Results
Plasma glucose and GA were elevated (29.7 ± 13.1 mmol/l [n = 5] and 33.3 ± 6.9% [n = 3], respectively) but HbA1c was within normal limits (5.4 ± 2.6% [35.5 ± 4.9 mmol/mol]; n = 4) at diagnosis. With diabetes treatment, aPPG (r = −0.565, p = 0.002), GA (r = −0.552, p = 0.003) and HbF (r = −0.855, p < 0.0001) decreased with age, whereas HbA1c increased (r = 0.449, p = 0.004). GA was strongly positively correlated with aPPG (r = 0.784, p < 0.0001), while HbA1c showed no correlation with aPPG (r = 0.221, p = 0.257) and was significantly inversely correlated with HbF (r = −0.539, p = 0.004).
Conclusions/interpretation
GA is a useful indicator of glycaemic control in patients with NDM, whereas HbA1c is influenced by age-related changes in HbF and does not accurately reflect glycaemic control.
Similar content being viewed by others
Introduction
Neonatal diabetes mellitus (NDM) is a type of diabetes that results from a single gene abnormality as a result of which insulin dependence develops within 6 months of birth [1]. NDM is clinically divided into transient NDM (TNDM), in which treatment is no longer required a few months after diagnosis, and permanent NDM (PNDM), in which lifelong treatment is required. TNDM is mainly caused by the overexpression of an imprinted region of chromosome 6q24 with paternal expression (i.e., paternal uniparental disomy of chromosome 6 [pUPD6], paternal 6q24 duplication, or methylation defect of maternal 6q24). In most cases, diabetes occurs within 1 week of birth, with remission in an average of 3 months [2, 3]. PNDM is mainly caused by an abnormality of the KCNJ11 gene that encodes the Kir6.2 subunit of the ATP-sensitive potassium channel of pancreatic beta cells, with onset at a mean age of 7 weeks [3, 4].
Glycation of various proteins is known to occur at higher rates in individuals with diabetes compared with those without diabetes. Some of these glycated proteins are thought to be involved in the onset and progression of chronic diabetic complications [5]. Of these glycated proteins, HbA1c is widely used as an indicator of glycaemic control [6]. Since the erythrocyte lifespan is approximately 120 days, HbA1c levels reflect the plasma glucose levels over the preceding 2–3 months. However, HbA1c levels are affected in some diseases that shorten the erythrocyte lifespan, such as haemolytic anaemia and renal anaemia, which are associated with lowered levels of HbA1c [7]. Furthermore, the accuracy of some HbA1c analysis methods is affected by the presence of variant haemoglobins [8]. Thus, HbA1c levels may not accurately reflect glycaemic control in these situations [8]. In addition to variant haemoglobin, some assays and calculation methods for estimating HbA1c indicate that HbA1c levels appear low when fetal haemoglobin (HbF) levels are high [9, 10]. HbF is the main haemoglobin present during the fetal period and accounts for 80–90% of haemoglobin just after birth, whereas haemoglobin A (HbA) accounts for only 10–20% [11]. After birth, HbF is gradually replaced by HbA; by 6 months of age, most of the haemoglobin present is HbA. Therefore, if HbA1c, a glycosylation product of HbA, is reported as a percentage of total haemoglobin, the influence of HbF in neonates leads to apparently low HbA1c. It is therefore difficult to use HbA1c as an indicator of glycaemic control in neonates. Glycation of any type of haemoglobin can be detected by affinity chromatography [12]. However, glycated haemoglobin levels appear to be low when HbF levels are high, because of the lower glycation rate of HbF compared with that of HbA [10]. Consequently, none of the current glycaemic indicators have been useful for patients with NDM; plasma glucose analysis is therefore currently used for the diagnosis and treatment of NDM.
Glycated albumin (GA) is also used as an indicator of glycaemic control [13]. Because the half-life of serum albumin is shorter than that of erythrocytes, GA reflects plasma glucose levels over a shorter period of time [14]. Thus, use of GA has been advocated for monitoring short-term changes in glycaemic control [15]. In addition, because GA is not affected by haemoglobin metabolism, it is a useful indicator of glycaemic control in patients with haemolytic anaemia [16]. Moreover, we have reported that, because GA is not affected by HbF, it can be used as an indicator of glycaemic control in neonates, when measured in umbilical cord blood [17]. Therefore, GA and HbA1c were compared in this study to evaluate their utility in monitoring glycaemic control in patients with NDM.
Methods
Study patients
This study included five patients with NDM (PNDM, 4; TNDM, 1) who were referred to the Department of Pediatrics, Asahikawa Medical University (Table 1). Their gestation period was 39 ± 1.7 weeks, and their birthweight was 2,069 ± 601 g. The birthweight SD score corrected for gestational age in Japanese babies [18], was low (−2.5 ± 0.9 SD). Age at diagnosis was 38 ± 20 days. Insulin therapy was started in all patients immediately after diagnosis. HbA1c and GA were measured in four and three patients, respectively, at the time of diagnosis. Three patients were referred immediately to the department, and the other two patients were referred later. Age at referral was 58 ± 11 days. After referral, HbA1c, GA and HbF were measured monthly for 6 months after diagnosis. Based on the results of self-monitoring of blood glucose (3.8 ± 2.3 times/day) before feeding, average preprandial plasma glucose (aPPG) levels were calculated for a 1 month period.
The KCNJ11 gene analysis was performed by direct sequencing in all patients [3]. Heterozygous mutations were identified in three patients (Patient 1, p.V59M; Patient 4, p.R201C; and Patient 5, p.F35V). Patient 2 had pancreatic hypoplasia (PH). Patient 3 exhibited TNDM and insulin therapy was discontinued at 5 months of age. We performed microsatellite marker analysis of chromosome 6 [3] on Patient 3, confirming that the patient had TNDM due to pUPD6. All steps of this study, including DNA analysis, were approved by the Ethics Committees at Asahikawa Medical University, and the study complied with the ethical guidelines of the Helsinki Declaration as revised in 2000. All patients’ parents provided written informed consent.
Laboratory methods
Plasma glucose at diagnosis was determined using a standard laboratory assay. HbA1c levels were determined by use of the following two methods: HPLC method using HLC-723G8 (Tosoh, Tokyo, Japan) for Patients 1, 3, and 5, and ADAMS-A1C HA-8160 (Arkray, Kyoto, Japan) for Patients 2 and 4. HbA1c is calculated as a percentage of total haemoglobin. The value for HbA1c (%) was estimated as a National Glycohaemoglobin Standardization Program (NGSP) equivalent value (%), calculated by the formula HbA1c (%) = HbA1c (Japan Diabetes Society: JDS) (%) +0.4%. This calculation takes into consideration the relationship between HbA1c (JDS) (%), measured by the previous Japanese standard substance and measurement methods, and HbA1c (NGSP) [19]. Normal control HbA1c (NGSP) levels for adults are in the range of 4.7–6.2%.
Serum GA was determined by an enzymatic method using an albumin-specific protease, ketoamine oxidase, and an albumin assay reagent (Lucica GA-L; Asahi Kasei Pharma, Tokyo, Japan) [20]. GA was hydrolysed to amino acids by an albumin-specific proteinase and then oxidised by ketoamine oxidase, producing hydrogen peroxide, which was measured quantitatively. Serum GA levels were calculated as the percentage of GA relative to total albumin. Normal control serum GA levels for adults are in the range 11.0–16.0%. Albumin was measured in the same serum sample using a new bromocresol purple method (Lucica GA-L; Asahi Kasei Pharma). Normal control serum albumin levels for adults are in the range 39–49 g/l with this method.
Statistical analysis
Results are expressed as means ± SD. Simple regression analyses were used to assess the associations between continuous variables. All analyses were performed using SPSS version 16.0 (SPSS, Chicago, IL, USA). p values were calculated and the level of significance was set at p < 0.05.
Results
At the time of diagnosis, plasma glucose and GA were markedly elevated in our study group (29.7 ± 13.1 mmol/l [n = 5] and 33.3 ± 6.9% [n = 3], respectively) (Table 1). On the other hand, HbA1c was elevated only in patient 1; it was within normal limits in patients 2 and 4 and was low in patient 3. The mean HbA1c was 5.4 ± 2.6% (35.5 ± 4.9 mmol/mol) (n = 4).
Figure 1 depicts the clinical course of patient 1 from the time of diagnosis. Patient 1, a boy born at 41 weeks of gestation weighing 2,582 g (−1.9 SD) at birth, exhibited poor feeding for several days before the first evaluation and was admitted to the local hospital at 53 days of age because he was ‘not doing well’. Laboratory tests at the initial evaluation showed the following: PG, 47.5 mmol/l; GA, 40.8%; HbA1c, 8.9% (73.8 mmol/mol); HbF, 22.2%; serum C-peptide level, <0.06 nmol/l; and pH, 6.826 on arterial blood gas analysis. Diabetic ketoacidosis was diagnosed, and insulin therapy was started immediately. With insulin therapy, his general condition improved and his aPPG and GA levels decreased. However, despite treatment for diabetes, HbA1c levels gradually increased until 5 months of age. After that point, HbA1c levels decreased. HbF levels decreased over time.
Clinical course of Patient 1. The time courses of aPPG (white circles), GA (black circles), HbA1c (black squares), and HbF (white squares) since diagnosis of diabetes in Patient 1 are shown. aPlasma glucose at diagnosis. To convert values for HbA1c in % to mmol/mmol, subtract 2.15 and then multiply by 10.929
Figure 2 depicts the clinical course (in other words, the relationship between diabetes treatment and age [days]) for aPPG, GA and HbA1c in all patients. aPPG was significantly inversely correlated with age as expected, given the course of treatment for diabetes (r = −0.565, p = 0.002). Similarly, GA was also significantly inversely correlated with age (r = -0.552, p = 0.003). On the other hand, HbA1c was significantly positively correlated with age (r = 0.449, p = 0.004). HbF also decreased with age, but at 7 months of age (215 ± 18 days), the value (3.2 ± 1.3%) was still higher than normal (<2%).
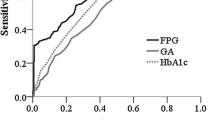
Next, we evaluated whether GA or HbA1c reflected glycaemic control in NDM patients. GA was strongly positively correlated with aPPG (r = 0.784, p < 0.0001) (Fig. 3). However, HbA1c showed no correlation with aPPG (r = 0.221, p = 0.257), and HbA1c was significantly inversely correlated with HbF (r = −0.539, p = 0.004) (Fig. 3).
Discussion
At the time of diagnosis, the mean HbA1c was within normal limits for all patients with NDM. In the neonatal period, the level of HbA1c is low [17] if HbA1c is calculated as a percentage of total haemoglobin, because HbF is the main haemoglobin and HbA accounts for only a small percentage of the total haemoglobin. In addition, most erythrocytes containing HbA in newborns are newly formed after birth and have a relatively short lifespan, which can add to the apparent low value of HbA1c. As a result, HbA1c was measured to be relatively low at the time of NDM diagnosis. However, HbA1c increased despite a decrease in aPPG levels after diabetes treatment. This paradoxical phenomenon may be explained in two ways. First, HbA levels increase as HbF decreases with age. Second, the lifespan of erythrocytes containing HbA increases with age. In patients with NDM, HbA1c levels did not correlate with aPPG, but inversely correlated with HbF. Our findings therefore confirmed that HbA1c is not suitable as a glycaemic control marker for patients with NDM when, as in this study, HbA1c levels are measured by HPLC.
Our study was limited in that we did not compare HbA1c levels obtained from a variety of methods. However, measurements using immunoassay and enzymatic assays tend to yield similar results, because they are both specific assays for HbA1c and do not measure HbF glycosylation products [17]. Although affinity methods can measure total glycated haemoglobins (including both HbA and HbF), glycated haemoglobin levels tend to be measured as low when levels of HbF are high. This is because the glycation rate for HbF, which does not have a glycation site at the N-terminal valine of the gamma chain, may be lower than the glycation rate of HbA [10]. In fact, we found that glycated haemoglobin, measured by an affinity method, did appear low in cord blood [17]. Therefore, these methods should not be used for the assessment of glycation levels in samples from infants. On the other hand, quantification by some HPLC methods (other than those used in this study) showed almost similar HbA1c level in samples with normal to higher than 20% HbF content [10]. These methods were, unfortunately, unavailable to us. There is a possibility that HbA1c may be a useful marker if it is calculated as HbA1c/(total haemoglobin − HbF) in order to eliminate the influence of high HbF levels in neonates. Although we did not examine this issue, the relationship between HbA1c/(total haemoglobin − HbF) and plasma glucose or GA should be examined in future.
Fructosamine and 1,5-anhydroglucitol (1,5-AG), neither of which are affected by haemoglobin metabolism, have also been used as indicators of glycaemic control in adults [21, 22]. However, because 1,5-AG is mainly ingested through food and almost no 1,5-AG is ingested during the neonatal period, serum 1,5-AG levels are undetectable in neonates [23]. Moreover, since fructosamine measures the amount of glycosylated protein, it is influenced by serum protein concentrations [24, 25]. Consequently, low fructosamine levels have been reported during the neonatal period, since serum proteins are low [25]. Moreover, total IgG in neonates, which is derived from maternal IgG, gradually disappears during the first 6–8 months of life. At the same time, the rate of infant IgG synthesis increases [26]. As a result, total IgG usually reaches a low point at 3–4 months of age. Because IgG—one of the major components of total protein—is easily glycated [27], the alteration of IgG in infancy may be a large contributor to alterations of fructosamine levels.
GA quantification is not affected by haemoglobin metabolism, nor is it affected by serum albumin concentrations, except in the case of high albumin metabolism, such as that which occurs in nephrotic syndrome [13], since GA is measured against serum albumin as a ratio [13, 16]. In fact, GA is much less affected by serum total protein concentration than is fructosamine [25]. In addition, Shima et al. reported that GA levels are not influenced by the concentration of total serum proteins [28]. In this study, GA was high at the time of NDM diagnosis. With diabetes treatment, GA decreased along with aPPG. Moreover, GA showed a strong positive correlation with aPPG. Based on these results, GA appears to be a very useful marker for both NDM diagnosis and treatment.
GA better reflects short-term changes in plasma glucose than does HbA1c [14, 15]. In patients with fulminant type 1 diabetes, a subtype of type 1 diabetes in which the progression from normoglycaemia to hyperglycaemia accompanied by ketoacidosis is extremely rapid [29], HbA1c levels at onset are normal or only slightly high [30]. On the other hand, GA and GA/HbA1c ratios are already high at disease onset [31]. Therefore, in patients with NDM, in whom hyperglycaemia occurs within a short period from birth to diagnosis, GA is already high at the time of NDM diagnosis—similarly to those individuals with fulminant type 1 diabetes. Moreover, since GA is a shorter-term glycaemic control marker, it may be more useful than a longer-term marker for patients with NDM; it is desirable to monitor glycaemic control changes more frequently in neonates.
We previously reported that the GA values in umbilical cord blood were 9.4 ± 1.1%, which was low compared with the reference values in adults. This may reflect low glucose levels or increased albumin metabolism in the fetus [17]. In this study, GA in infants with NDM was found to be higher than the normal reference values for adults. Abe et al. [25] reported GA reference values for infants, but in that study, GA was measured using HPLC. The use of enzymatic methods, rather than HPLC, is known to provide lower GA values [20], but GA is most commonly measured using an enzymatic method. In the future, GA reference values for infants, measured using enzymatic methods, need to be established.
In conclusion, this study has shown that GA can be a useful indicator of glycaemic control in patients with NDM. These findings will need to be further confirmed in a larger number of patients with NDM.
Abbreviations
- 1,5-AG:
-
1,5-Anhydroglucitol
- aPPG:
-
Average preprandial plasma glucose
- GA:
-
Glycated albumin
- HbA:
-
Haemoglobin A
- HbF:
-
Fetal haemoglobin
- NDM:
-
Neonatal diabetes mellitus
- JDS:
-
Japan Diabetes Society
- NGSP:
-
National Glycohaemoglobin Standardization Program
- PH:
-
Pancreatic hypoplasia
- PNDM:
-
Permanent NDM
- pUPD6:
-
Paternal uniparental disomy of chromosome 6
- TNDM:
-
Transient NDM
References
Aguilar-Bryan L, Bryan J (2008) Neonatal diabetes mellitus. Endocr Rev 29:265–291
Temple IK, Gardner RJ, Mackay DJ et al (2000) Transient neonatal diabetes: widening the understanding of the etiopathogenesis of diabetes. Diabetes 49:1359–1366
Suzuki S, Makita Y, Mukai T et al (2007) Molecular basis of neonatal diabetes in Japanese patients. J Clin Endocrinol Metab 92:3979–3985
Gloyn AL, Pearson ER, Antcliff JF et al (2004) Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med 350:1838–1849
Cohen MP (1998) Nonenzymatic glycation: a central mechanism in diabetic microvasculopathy? J Diabet Complications 2:214–217
Koenig RJ, Peterson CM, Jones RL et al (1976) Correlation of glucose regulation and haemoglobin AIc in diabetes mellitus. N Engl J Med 295:417–420
Jeffcoate SL (2004) Diabetes control and complications: the role of glycated haemoglobin, 25 years on. Diabet Med 1:657–665
Bry L, Chen PC, Sacks DB (2001) Effects of haemoglobin variants and chemically modified derivatives on assays for glycohaemoglobin. Clin Chem 47:153–163
Felner EI, McGrath M (2008) Inaccurate haemoglobin A1C levels in patients with type 1 diabetes and hereditary persistence of haemoglobin F. J Pediatr 153:137–139
Rohlfing CL, Connolly SM, England JD et al (2008) The effect of elevated fetal haemoglobin on haemoglobin A1c results: five common haemoglobin A1c methods compared with the IFCC reference method. Am J Clin Pathol 129:811–814
Ohls RK, Christensen RD (2008) Developmental of the hematopietic system. In: Kliegman RM, Beheman RE, Jenson HB, Santon BF (eds) Nelson textbook of pediatrics, 18th edn. Saunders Elsevier, Philadelphia, pp 1997–2003
Chapelle JP, Teixeira J, Maisin D et al (2010) Multicentre evaluation of the Tosoh HbA1c G8 analyser. Clin Chem Lab Med 48:365–371
Koga M, Kasayama S (2010) Clinical impact of glycated albumin as another glycemic control marker. Endocr J 57:751–762
Tahara Y, Shima K (1995) Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care 18:440–447
Takahashi S, Uchino H, Shimizu T et al (2007) Comparison of glycated albumin (GA) and glycated haemoglobin (HbA1c) in type 2 diabetic patients: usefulness of GA for evaluation of short-term changes in glycemic control. Endocr J 54:139–144
Koga M, Hashimoto K, Murai J et al (2011) Usefulness of glycated albumin as an indicator of glycemic control status in patients with hemolytic anemia. Clin Chim Acta 412:253–257
Koga M, Murai J, Saito H et al. (2011) Measurement of glycated haemoglobin and glycated albumin in umbilical cord: evaluation of the glycemic control indicators in neonates. J Perinatol 6:430–433
Nishida H, Sakamoto S, Sakanoue M (1985) New fetal growth curves for Japanese. Acta Paediatr Scand Suppl 319:62–67
The Committee of Japan Diabetes Society on the diagnostic criteria of diabetes mellitus (2010) Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. J Diabetes Invest 1:212–228
Kouzuma T, Usami T, Yamakoshi M et al (2002) An enzymatic method for the measurement of glycated albumin in biological samples. Clin Chim Acta 324:61–71
Armbruster DA (1987) Fructosamine: structure, analysis, and clinical usefulness. Clin Chem 33:2153–2163
Yamanouchi T, Akanuma Y (1994) Serum 1,5-anhydroglucitol (1,5 AG): new clinical marker for glycemic control. Diabetes Res Clin Pract 24(Suppl):S261–S268
Yoshioka S (1983) A new metabolic parameter, 1-deoxyglucose (1,5-anhydroglucitol), for diabetes mellitus. Shohnika 24:405–410 (in Japanese)
Senecal PE, Douville P, Simard S et al (1988) The relationship between serum fructosamine and albumin. Clin Chim Acta 173:239–241
Abe F, Yano M, Minami Y et al (1989) Alterations in fructosamine and glycated albumin levels during childhood. Ann Clin Biochem 26:328–331
Buckley RH (2008) T lymphocytes, B lymphocytes, and natural killer cells. In: Kliegman RM, Beheman RE, Jenson HB, Santon BF (eds) Nelson textbook of pediatrics, 18th edn. Saunders Elsevier, Philadelphia, pp 873–879
Danze P, Tarjoman A, Rousseaux J et al (1987) Evidence for an increased glycation of IgG in diabetic patients. Clin Chim Acta 166:143–153
Shima K, Ito N, Abe F et al (1988) High-performance liquid chromatographic assay of serum glycated albumin. Diabetologia 31:627–631
Hanafusa T, Imagawa A (2007) Fulminant type 1 diabetes: a novel clinical entity requiring special attention by all medical practitioners. Nat Clin Pract Endocrinol Metab 3:36–45
Imagawa A, Hanafusa T, Uchigata Y et al (2003) Fulminant type 1 diabetes: a nationwide survey in Japan. Diabetes Care 26:2345–2352
Koga M, Murai J, Saito H et al (2010) Serum glycated albumin to haemoglobin A1C ratio can distinguish fulminant type 1 diabetes mellitus from type 2 diabetes mellitus. Ann Clin Biochem 47:313–317
Acknowledgements
This study was supported by a Grant-in-Aid for Young Scientists (B) (21790965) provided by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
S.S. and M.K. researched data, contributed to the discussion, wrote the manuscript, and reviewed/edited the manuscript. S.A., A.N., K.W., K.O., S.H. and A.R.S. researched data and drafted the manuscript. H.T., K.M. and Y.T. contributed to the discussion, and drafted and reviewed/edited the manuscript. K.F. supervised the research. S.S., M.K., S.A., A.N., K.W., K.O., S.H., A.R.S, H.T., K.M. and Y.T. approved the final version.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Professor K. Fujieda, who supervised this research, died on 19 March 2010 before publication of this work.
Rights and permissions
About this article
Cite this article
Suzuki, S., Koga, M., Amamiya, S. et al. Glycated albumin but not HbA1c reflects glycaemic control in patients with neonatal diabetes mellitus. Diabetologia 54, 2247–2253 (2011). https://doi.org/10.1007/s00125-011-2211-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-011-2211-8