Abstract
Aims/hypothesis
Exendin-4 is a 39 amino acid agonist of the glucagon-like peptide receptor and has been approved for treatment of type 2 diabetes. Many reports describe an increased incidence of acute pancreatitis in humans treated with exendin-4 (exenatide). Previous studies have evaluated the effect of exendin-4 on beta cells and beta cell function. We evaluated the histological and biochemical effects of exendin-4 on the pancreas in rats.
Methods
We studied 20 Sprague–Dawley male rats, ten of which were treated with exendin-4 and ten of which were used as controls. The study period was 75 days. Serum and pancreatic tissue were removed for biochemical and histological study. Blood glucose, amylase, lipase, insulin and adipocytokines were compared between the two groups.
Results
Animals treated with exendin-4 had more pancreatic acinar inflammation, more pyknotic nuclei and weighed significantly less than control rats. They also had higher serum lipase than control animals. Exendin-4 treatment was associated with lower insulin and leptin levels as well as lower HOMA values than in the untreated control group.
Conclusions/interpretation
Although the use of exendin-4 in rats is associated with decreased weight gain, lower insulin resistance and lower leptin levels than in control animals, extended use of exendin-4 in rats leads to pancreatic acinar inflammation and pyknosis. This raises important concerns about the likelihood of inducing acute pancreatitis in humans receiving incretin mimetic therapy.
Similar content being viewed by others
Introduction
Exendin-4 (Exenatide) is a 39-amino acid agonist of the glucagon-like peptide (GLP-1) receptor that is approved as an adjunctive treatment for type 2 diabetes [1]. The compound was originally isolated from the saliva of Heloderma suspectum [2], a lizard that eats only three to four times per year and must digest its food very slowly. GLP-1 is a gastrointestinal hormone, which regulates blood glucose primarily by stimulating glucose-dependent insulin release [3]. Exendin-4 is a long-acting agonist of the GLP-1 receptor [4].
In April 2005 the United States Food and Drug Administration (FDA) approved the use of exenatide as adjunctive therapy to improve glycaemic control in patients with type 2 diabetes [5]. There have since been many reports associating acute pancreatitis with the use of exenatide. Thus from the date of approval through to 31 December 2006, 48 domestic cases of acute pancreatitis associated with exenatide therapy were reported to the FDA and entered in the FDA’s Adverse Event Reporting System database [5]. In October 2007, the FDA added an alert for healthcare professionals after reviewing 30 post-marketing reports on patients who developed acute pancreatitis while using [6]. Following this healthcare warning in August 2008, the FDA received reports of six cases of haemorrhagic or necrotising pancreatitis leading to two deaths in patients receiving exenatide [6]. However, the effect of exendin-4 on pancreatic acinar cells and its role in causing pancreatitis is unknown. The aim of this study was to evaluate the histological and biochemical effects of exendin-4 on the pancreas in a rat model.
Methods
Animals
Experiments were approved by and performed in accordance with the University Animal Care and Use Committee in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-approved facility. We used 23 ~8-week-old outbred Sprague–Dawley male rats (Charles Rivers Labs, Wilmington, MA, USA), of which ten served as controls and ten were treated with exendin-4; the other three were used as procedure controls and killed at the start of the experiment. Animals were housed in individual cages in a room maintained at constant temperature and humidity on a 12 h light–dark cycle throughout the experimental period, with free access to chow and water. Chow was from Harlan (Teklad TD94149; Harlan Laboratories, Madison, WI, USA) and had defined amounts of complete minerals and vitamins, 15.4% of energy as protein, 63.1% as carbohydrate and 10% as fat.
Exendin-4 administration and tissue removal
Highly purified drug (exendin-4; ChemPep, Miami FL, USA) was stored at −70°C and dosages prepared as needed. In line with previous publications on exendin-4 in rats and in order to better elucidate the effect of exendin-4 on the pancreas, it was decided to use a dose of 10 μg/kg [7]. This dosage was administered subcutaneously to the treated group each day immediately before the 12 h dark cycle when rats are known to feed. Animal weights were recorded weekly and doses adjusted according to weight. The ten exendin-treated rats and ten control animals were killed after 75 days of treatment. Serum was obtained from each animal and necropsy tissue collection specimens were fixed in 10% formalin.
Histology
At the time of death, pancreases were immediately placed in 10% buffered formalin (4% [vol./vol/] formaldehyde). Specimens from each animal were paraffin-embedded and the 4 μm thick sections stained by haematoxylin and eosin. The degree of injury visible by light microscopy was scored by two researchers, who were not aware of the slide identification, according to a technique previously described and applied by one of the researchers to lungs, kidney and pancreas of experimental animals [8–10].
Organ damage was assessed by evaluating changes in the pancreatic acini, the ducts, the arteries (with particular attention to those of small calibre) and the islets of Langerhans. A subjective rating for each slide ranging from 5 (minimal) to 40 (severe and extensive damage) was assigned to each component of the organ. In the previous publications, the components of the histopathological scoring system were strongly correlated [8]. Acinar damage was evaluated according to appearance of each cell, its pyramidal structure, the regular patency of the acinar lumen, the presence of inflammatory cells, the number of pyknotic nuclei and the amount of intraluminal secretion of zymogenic granules. The severity of vasculitis was evaluated according to thickening of the medial wall, reduction of the lumen, intima hypertrophy and presence of inflammatory cells in the adventitia, and presence of intraluminal haemorrhages. The ductal changes reflected a more irregular disposition of the epithelial cells and the presence both of oedema in the wall and of inflammation. Damage to the islets of Langerhans was evaluated by the presence or absence of nuclei, the presence of intra-islet haemorrhages, nuclear pyknosis and inflammation.
Biochemical analyses
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were assayed by manual kinetic assay (Catachem, Bridgeport, CT, USA). Serum adiponectin was measured with a rat specific ELISA (Alpco Diagnostics, Salem, NH, USA). Serum insulin and leptin were measured on ultra-sensitive rat-specific ELISA kits (Crystal Chem, Downers Grove, IL, USA). Serum cholesterol and triacylglycerol levels were quantified by fluorescent and colorimetric ELISA assays respectively (Cayman Chemical, Ann Arbor, MI, USA). Serum α-amylase and lipase were measured by colorimetric enzyme assays (BioAssay Systems, Hayward, CA, USA). To provide quantitative measures of early apoptotic change (suggested by our histopathology findings of mild injury), caspase 9 was measured in pancreatic tissue supernatant fractions using a colorimetric microplate assay (R&D Systems, Minneapolis MN, USA).
Serum was frozen in aliquots at necropsy, stored at −70°C and thawed for each assay. Assay analyses were performed according to manufacturers’ instructions and evaluated with a fluorescent plate reader (Biotek PowerWave X colorimetric; FLX800, Bio-Tek Instruments, Winooski, VT, USA) with concentrations calculated by KC4 software. Homeostasis model assessment was calculated using previously described methods for assessment of insulin resistance [11].
Statistical analysis
The data are presented as mean ± SE. Statistical analysis was performed using Statistica software (StatSoft, Tulsa, OK, USA). Groups were compared using unpaired t tests or ANOVA and post tests. A p value of less than 0.05 was considered to be statistically significant.
Results
Effect of exendin-4 on weight of rats
At the end of the study, there was a significant difference in the weight of exendin-4-treated rats compared with control rats (p < 0.001, t test). Animals treated with exendin-4 had an approximately 30% reduction in weight compared with control animals (408.6 ± 43.4 vs 579.7 ± 78.05 g respectively) (Fig. 1).
Association of the use of exendin-4 with body weight over the period of the study. Rats were weighed at weekly intervals. There was a significant difference in body weight of rats in the two groups over the period of the study (9 weeks; n = 10 per group). Symbols shown the mean body weight; boxes show the deviation (SE); vertical lines show SD; dashed line shows the weight at the start
Effect of exendin-4 on glucose, insulin, liver enzymes and adipocytokines
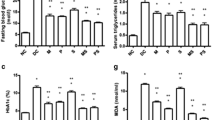
At the end of the study, animals treated with exendin-4 had higher AST levels than control rats. There was no statistical difference between ALT levels (Table 1). Rats in the exendin-4 group also had lower insulin, adiponectin and leptin levels, as well as lower HOMA values than rats in the control group (Table 1).
Serum amylase and lipase in control and exendin-4-treated rats
Direct assays of amylase and lipase activity in serum were performed in 96-well plates (BioAssay Systems). α-Amylase and lipase are important exocrine pancreatic enzymes. The group receiving exendin-4 had a serum amylase value of 162.4 ± 83.9 U/l, whereas controls had an amylase value of 201.88 ± 69.6 U/l, a non-significant difference (p = 0.27). Serum lipase was significantly increased in the exendin-4 group compared with controls (0.46 ± 0.12 and 0.28 ± 0.07 U/l respectively, p = 0.001) (Fig. 2).
Serum amylase (black bars) and lipase (white bars) levels in control and exendin-4 rats, showing SD (vertical bars). Serum from both groups was assayed by quantitative colorimetric enzyme tests. Serum α-amylases cleave the alpha-1-4 glycosidic bonds of starch to produce glucose and are usually increased in pancreatitis. The group receiving exendin-4 produced 162.4 ± 83.9 U/l amylase, whereas controls produced 201.88 ± 69.6 U/l, a non-significant difference (p = 0.27). Serum lipase, shown magnified by 100 to fit on same graph, was significantly increased in the exendin-4 group compared with controls (0.46 ± 0.12 and 0.28 ± 0.07 U/l respectively, p = 0.001). Lipase, the key enzyme for converting triacylglycerol to NEFA, usually increases dramatically (five- to tenfold) with pancreatitis. Values are means with standard deviations shown by vertical bars
Effect of exendin-4 on pancreatic cells
Figure 3 is a graphic representation of the pathology scores for five cellular variables analysed in sections of the pancreas of the two groups studied. The animals treated with exendin-4 had significantly more inflammation (p = 0.005) (particularly arteriolar vasculitis) and higher numbers of pancreatic pyknotic nuclei (p = 0.028, ANOVA and post tests) compared with the control animals. The lumen patency of five vessels for each animal was counted at a magnification of ×400. Patency was defined as the number of vessels (arteries and arterioles) with more than 50% lumen occlusion and adventitial presence of inflammatory cells, monocytes and lymphocytes. Control level of occlusion was 2.1 ± 1.85 and that of exendin-4-treated rats was 5.4 ± 2.37, the difference being significant (p = 0.003). The individual variances in scores from the groups yielded no statistically significant data in levels of pancreatic fibrosis, parenchymal structure or islet size, although subtle differences were broadly detected.
Statistical evaluation of pathology scores: summation for control (white bars) and exendin-4 (black bars) groups. Tissue sections of control (n = 10) and exendin-4-treated (n = 10) animals were evaluated by two pathologists unaware of the identity of the slides. Statistically significant differences between the two groups were observed for inflammation (p = 0.005) and the presence of pyknotic nuclei (p = 0.028) only
Caspase-9 in control and exendin-4-treated rats
Caspase-9 activity showed a borderline significant difference between the control and the exendin-4 group pancreases (p = 0.054), with a higher level of pro-apoptotic caspase-9 in the exendin-4 group (0.249 ± 0.1) than in controls (0.154 ± 0.08). These data corroborated our findings of mild histopathology as a result of 75 days treatment with the drug (Fig. 4).
Quantification of caspase-9 staining of the pancreas homogenate in control and exendin-4-treated animals. Caspase-9 activity was assessed in pancreas homogenate by microplate colorimetric assay to assess for cellular apoptosis. Values are mean (horizontal line) ± SD (box) and SD (vertical bars). There was a borderline significant difference (p = 0.054) between the control and the exendin-4 group pancreases, corroborating our findings of mild histopathology as a result of 75 days treatment with the drug
Figure 5a–f demonstrates sections of rat pancreas stained with haematoxylin and eosin at magnifications of ×100 or ×400. Pancreatic acinar cells of exendin-4-treated rats had significant variation in size and shape (Fig. 5c, d), with most of the cells being smaller and irregular compared with cells of controls (Fig. 5e, f), which retained their regular pyramidal structure and expected intense basophilic staining. In Fig. 5d, a portion of the pancreas of an exendin-4-treated animal shows destruction of the acinar structure with inflammation and fibrosis. The lumens of the acinar glands of rats receiving exendin-4 were also larger than those of controls and secretion was more intensively eosinophilic (Fig. 5c). The acinar cells of exendin-4 rats also had a discrete number of pyknotic nuclei and nuclei presenting only as small aggregates of chromatin (Fig. 5b), while nuclei of the cells of control animals were larger in size with diffuse basophilic staining and very limited pyknosis (Fig. 5e, f).
Representative photographs of pancreatic tissue sections in rats treated with exendin-4 and controls. Sections were stained with haematoxylin and eosin. a Exendin-4-treated, magnification ×400. Vascular thickening (arrow) was seen in exocrine pancreas. Lumen patency, defined as number of arteries/arterioles with over 50% occlusion, was significantly increased with exendin-4 vs controls and more inflammatory cells were present in the adventitia. b Exendin-4-treated, ×400. Acinar structure disruption and pyknotic nuclei at arrows. No significant damage in islets of Langerhans. c Exendin-4-treated ×400. More severe acinar structure disruption involving large acinar section. d Exendin-4-treated ×100. Severe acinar destruction and fibrosis. e, f Controls ×400. No damage, acinar pancreas and islets of Langerhans
Discussion
Exendin-4 (exenatide) mimics the action of the incretin hormone GLP-1, which is released during meals from the gut endocrine cells [12]. Its beneficial glucoregulatory effects include enhancement of glucose-dependent insulin secretion, restoration of first-phase insulin response and suppression of inappropriately elevated glucagon secretion [12]. This beneficial role forms the basis for exenatide’s use in the treatment of type 2 diabetes. Interestingly, the major mechanism of exenatide’s action appears to be through its interaction with pancreatic cells. Recent reports suggest that exenatide has adverse pancreatic effects in some patients, triggering an FDA alert [6].
This study’s pathology data, by evaluating pancreatic tissue, suggest that exendin-4 is a significant factor in producing pancreatic inflammation as well as increased numbers of pyknotic cells in pancreatic acinar cells of exendin-4-treated rats compared with control rats. Pyknosis is the irreversible condensation of the chromatin in the nucleus of a cell undergoing programmed cell death or apoptosis. Pancreatic effects were also confirmed biochemically in this study, with exendin-4-treated rats demonstrating significantly higher lipase values as than controls. This information is valuable given the recent increase in the number of patients experiencing acute pancreatitis associated with the use of exenatide. Measurement of serum α-amylase and lipase, both highly important exocrine pancreatic enzymes, produced uneven results with regard to pancreatic function in the exendin-4 group as compared with controls. Thus α-amylase, which is usually increased in pancreatitis, showed a small but insignificant decrease in the exendin-4 group. However, serum lipase, which is a more specific marker of acute pancreatitis [13] and is expected to rise significantly with acute pancreatitis, increased about twofold in the exendin-4 group. Additionally, we observed increased caspase-9 activity in rats receiving exendin-4. Caspase-9 is an essential initiator caspase [14] for the apoptotic effector caspases such as caspase 3.
There are several possible explanations for the association of pancreatitis with the use of exendin-4. One recent study on exendin-4 and GLP-1 suggested that these products stimulate exocrine and endocrine pancreatic secretion through local and central neurons of the vagus, offering a broad spectrum of effects on the pancreas [15]. It has also been reported that the biological product (exenatide purified from Heloderma suspectum venom) caused pancreatitis in humans [5], although the level of purification of that product was not available. Another explanation put forth is that the lipoprotein and endocrine changes leading to weight loss may contribute to pancreatic effects. Exendin-4 is known to cause human weight loss [12], and indeed, the animals in the present study lost significant weight with exendin-4. In another study by Koehler et al., the authors found that GLP-1 receptor activation increased the pancreatic mass and selectively modulated expression of genes associated with pancreatitis [16]. Also, in a study looking at sitagliptin, an inhibitor of dipeptidyl peptidase-4, an enzyme that breaks down incretin hormones, sitagliptin treatment was associated with increased pancreatic ductal turnover, ductal metaplasia and in one rat pancreatitis [17]. In that study, although ductal GLP-1 receptor expression was not altered, it was postulated that the pancreatic effects could be related to increased GLP-1 concentrations [17].
Although we observed pancreatic nuclear changes as discussed above, no overall significant difference in the parenchymal structure, fibrosis or islet cell size was seen in pancreatic cells of exendin-4-treated rats vs controls. In a study looking at the islet cell size of animals treated with GLP-1 agonists in a mouse model, it was found that after 2 weeks of treatment islet cell size increased 1.7-fold in mice treated with GLP-1 agonists [18,19]. However, in our study an extended period of exendin-4 administration was used and the animal model used was a rat, not a murine model. The reason for the increased number of pyknotic cells and pancreatic inflammation observed by us in rats treated with exendin-4 is not clear. Pyknotic cells, i.e. cells that may be progressing to apoptosis, are in some way stressed or responding to a physiological change. In the early studies on exendin, it was found that exendin-3, which was derived from the saliva of H. horridum, stimulated amylase release [19,20]. However, this effect was not present with exendin-4 at concentrations of 1 μmol/l, although there was only a difference of two amino acid substitutions between the two peptides [20]. Nevertheless, in later studies it was found that combining exendin-4 with agents that increased cell calcium resulted in a potentiation of amylase release [20].
The histology demonstrated in Fig. 5 shows that the differences between controls and exendin-4-treated animals were not very striking. A slight difference in size and shape of the exocrine pancreatic cells and a more intense eosinophilic staining of the cellular secretion were counterweighed by a more intense basophilic stain in the pancreatic acinar cells of the controls. There were more pyknotic nuclei and evidence of moderate fibrotic changes in the vascular and the ductal walls of rats receiving exendin-4. Changes in the histological appearance of the islets of Langerhans were less striking. The changes described above, however, were repeatedly and systematically observed in the tissue sections of control and exendin-4-treated animals and recorded by two pathologists (A. Molteni and a resident pathologist), who were unaware of the identity of the slides.
The animals in the present study demonstrated about 30% less weight gain with exendin-4 as compared with controls, a finding that is similar to those of Ding et al. [7]. Also, in our study, rats treated with exendin-4 had lower insulin, adiponectin and leptin levels than control rats. Previous studies have found that adiponectin possibly plays a protective role in acute pancreatitis [21]. In our study, we found that the rats treated with exendin-4 had lower serum adiponectin than control rats, albeit not statistically significant. It is possible that the effect of exendin-4 on adiponectin may contribute to the effect of exendin-4 on pancreatic acinar cells. Thus, it would seem that even if administration of exendin-4 improved insulin resistance in rats and reduced inflammatory markers like leptin, it nevertheless had a deleterious effect on pancreatic histology, leading to moderate inflammation and pyknosis. Our observations may offer, at a cellular level, some clarification of reports of pancreatitis observed in patients taking exenatide.
It can be argued that the effects of exendin-4 observed in rodents may not be applicable to humans. However, given the recent increase of pancreatitis in patients using exenatide, this study does raise concerns about whether this incretin mimetic induces pancreatic modification. An article by Bain et al. [22] described studies done by Lilly Research Laboratories in rats, mice and monkeys, in which no target organ damage was observed. However, these data have never been published.
In summary, although the use of exendin-4 in rodents is associated with decreased weight gain, lower insulin resistance and lower leptin levels than in control rats, the extended use of exendin-4 in rats leads to pancreatic acinar inflammation and pyknosis. These data raise important concerns about the pancreatic effects of incretin mimetics, which, given their wide clinical use, should be clarified with urgency.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- FDA:
-
Food and Drug Administration
- GLP:
-
Glucagon-like peptide
References
Denker PS, Dimarco PE (2006) Exenatide (exendin-4)-induced pancreatitis: a case report. Diabetes Care 29:471
Egan JM, Clocquet AR, Elahi D (2002) The insulinotropic effect of acute exendin-4 administered to humans: comparison of nondiabetic state to type 2 diabetes. J Clin Endocrinol Metab 87:1282–1290
De Leon DD, Crutchlow MF, Ham JY, Stoffers DA (2006) Role of glucagon-like peptide-1 in the pathogenesis and treatment of diabetes mellitus. Int J Biochem Cell Biol 38:845–859
Goke R, Fehmann HC, Linn T et al (1993) Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting beta-cells. J Biol Chem 268:19650–19655
Ahmad SR, Swann J (2008) Exenatide and rare adverse events. N Engl J Med 358:1970–1971 discussion 1971–1972
FDA U.S. Food and Drug Administration (2007) Byetta (exanatide) October 2007. Available from www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm150839.htm, accessed 21 November 2008
Ding X, Saxena NK, Lin S, Gupta NA, Anania FA (2006) Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 43:173–181
Reddy MK, Baskaran K, Molteni A (1995) Inhibitors of angiotensin-converting enzyme modulate mitosis and gene expression in pancreatic cancer cells. Proc Soc Exp Biol Med 210:221–226
Molteni A, Bahu RM, Battifora HA et al (1979) Estradiol receptor assays in normal and neoplastic tissues. A possible diagnostic acid for tumor differentiation. Ann Clin Lab Sci 9:103–108
Molteni A, Moulder JE, Cohen EP et al (2001) Prevention of radiation-induced nephropathy and fibrosis in a model of bone marrow transplant by an angiotensin II receptor blocker. Exp Biol Med (Maywood) 226:1016–1023
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Triplitt C, Chiquette E (2006) Exenatide: from the Gila monster to the pharmacy. J Am Pharm Assoc (2003) 46:44–52 quiz 53–54
Carroll JK, Herrick B, Gipson T, Lee SP (2007) Acute pancreatitis: diagnosis, prognosis, and treatment. Am Fam Physician 75:1513–1520
Chen M, Wang J (2002) Initiator caspases in apoptosis signaling pathways. Apoptosis 7:313–319
Wan S, Coleman FH, Travagli RA (2007) Glucagon-like peptide-1 excites pancreas-projecting preganglionic vagal motoneurons. Am J Physiol Gastrointest Liver Physiol 292:G1474–1482
Koehler JA, Baggio LL, Lamont BJ, Ali S, Drucker DJ (2009) Glucagon-like peptide-1 receptor activation modulates pancreatitis-associated gene expression but does not modify the susceptibility to experimental pancreatitis in mice. Diabetes 58:2148–2161
Matveyenko AV, Dry S, Cox HI et al (2009) Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin. Diabetes 58:1604–1615
Stoffers DA, Kieffer TJ, Hussain MA et al (2000) Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes 49:741–748
Eng J (1992) Exendin peptides. Mt Sinai J Med 59:147–149
Malhotra R, Singh L, Eng J, Raufman JP (1992) Exendin-4, a new peptide from Heloderma suspectum venom, potentiates cholecystokinin-induced amylase release from rat pancreatic acini. Regul Pept 41:149–156
Araki H, Nishihara T, Matsuda M et al (2008) Adiponectin plays a protective role in caerulein-induced acute pancreatitis in mice fed a high-fat diet. Gut 57:1431–1440
Bain SC, Stephens JW (2008) Exenatide and pancreatitis: an update. Expert Opin Drug Saf 7:643–644
Acknowledgements
The authors acknowledge the generous support of the Saint Luke’s Hospital Foundation, Kansas City, MO, USA, for their funding of this study. The authors would also like to acknowledge the help of D. Campbell (University of Missouri Kansas City School of Medicine, Kansas City, MO, USA) for his valuable suggestions and reviewing and proofreading of this manuscript.
Duality of interest
D. Bulchandani owns stocks in Eli-Lilly Pharmaceuticals. The authors declare that there is no other duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nachnani, J.S., Bulchandani, D.G., Nookala, A. et al. Biochemical and histological effects of exendin-4 (exenatide) on the rat pancreas. Diabetologia 53, 153–159 (2010). https://doi.org/10.1007/s00125-009-1515-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-009-1515-4